Abstract
Buprenorphine is a potent analgesic commonly administered to alleviate pain in sheep used in research. Sustained-release buprenorphine (SRB) is an alternative to conventional buprenorphine hydrochloride (which must be injected repeatedly). To compare SRB with a typical conventional buprenorphine regimen (0.03 mg/kg every 8 h for 72 h), we used a simple 1:1 conversion to calculate a total SRB dose of 0.27 mg/kg per injection. The pharmacokinetics and thermal nociceptive effects of SRB were analyzed in 4 healthy adult sheep after a single intramuscular injection plus a washout period then a single subcutaneous injection. For both routes in all 4 sheep, plasma buprenorphine concentrations exceeded 0.1 ng/mL, considered the minimal threshold for therapeutic benefit, after 12 h and maintained a steady state for at least 72 h Likewise, for both routes in all sheep, thermal thresholds increased significantly between baseline and 12 h; lack of response persisted for at least 72 h. The average maximal plasma buprenorphine concentrations and bioavailability were similar for both routes. No clinical adverse effects occurred. Using a dose equivalent to the total course of conventional buprenorphine, this pilot study suggests that SRB is a well-tolerated, effective, and long-acting analgesic that can be administered as a single intramuscular or subcutaneous injection. SRB confers steady plasma concentrations and continuous analgesia in thermal nociception for at least 72 h. When compared with conventional buprenorphine, SRB has considerable advantages in improving wellbeing by minimizing handling-associated stress of repeated injection and limiting the likelihood of end-of-dose breakthrough pain.
Abbreviation: SRB, sustained-release buprenorphine
To study disease and to develop and evaluate therapeutic interventions, researchers often heavily rely on animal models. Avoiding or minimizing pain in laboratory animals is of central importance to protect their wellbeing.2,37 The administration of analgesics, which block or reduce sensitization of central and peripheral pain pathways, is a key antinociceptive method.
Sheep are used to model interventions targeting airway disease, cardiovascular disease, orthopedic injury, emergency resuscitation, and vaccination.9,18,22,23,25,26,34,36,38,39 The development of effective analgesic regimens in sheep requires consideration of the source of pain, its anticipated duration, and the advantages and disadvantages of various methods.
Buprenorphine, a partial μ opiate agonist, is among the most commonly used analgesics in laboratory animals, including sheep.1,19,28,42 It is used primarily as an injectable and sublingual analgesic to alleviate mild to severe pain.32 Favored for its relatively long half-life and exceptional safety profile, buprenorphine has several other advantageous properties: rapid onset, higher potency than morphine, and a ceiling in terms of its respiratory effect but not its analgesic effect.6,31 Its analgesic effect usually lasts for 6 to 8 h, but may persist for as long as 12 h, depending on the type of pain.32 The duration of effect and the response to buprenorphine are also related to species-specific metabolism and are dose-dependent.12
Sustained-release buprenorphine (SRB) is approved for veterinary analgesic use. It offers prolonged pain relief, minimizing the need for repeated injections. Its efficacy has been demonstrated in mice, rats, cats, and NHP under different applications, including surgical models.3,4,8,29 Similarly, extended-release opioid formulations are used clinically in humans, prolonging pain relief, lengthening dose intervals, reducing end-of-dose pain, and enhancing ease of compliance.16,17,27,41 In sheep, limiting the need for repeated injections (which typically must occur every 4 to 12 h) is especially appealing, considering their susceptibility to stress during flock separation and under restraint when the prey flight response is blocked.
Because SRB is a relatively new formulation, the dose basis and ideal route of administration have yet to be established in sheep. The purpose of this study was to characterize the pharmacokinetic profile of SRB, in relation to its analgesic efficacy, as measured by thermal threshold and safety parameters. Identifying the expected plasma concentrations of buprenorphine after SRB administration allows these data to be compared with previous reported findings for conventional buprenorphine.28,42 Our hypothesis, based on assumptions from similar veterinary applications, was that parenteral injection of SRB every 72 h would result in therapeutic concentrations and provide continuous analgesia. Using a crossover study design, we administered SRB through a single intramuscular and then a single subcutaneous injection; we measured the response to thermal nociception and the plasma concentrations at serial time points for 7 d. In addition, we monitored sheep for side effects associated with the SRB dose and the route of administration, particularly cardiovascular or respiratory suppression and inappetence.
Our hope was that the pharmacokinetic profile of SRB would provide advantages such as steady-state plasma concentrations, decreased end-of-dose failure, and minimization of injection-related handling stress. We believe that a preliminary characterization of the pharmacokinetic profile of SRB and of the response to this drug is essential to develop effective dose regimens for practical application.
Materials and Methods
The IACUC at the University of Minnesota approved this study.
Animals.
Four naïve Suffolk cross sheep, 2 wethers (sheep 1: age, 4 y; sheep 2: age, 7 y) and 2 ewes (sheep 3: age, 2 y; sheep 4: age, 6 y) were used for this pilot study. Sheep were immunized with Clostridium perfringens types C and D and tetanus toxoid yearly and given anthelmintics (dewormed) twice annually (spring and fall). All 4 were group-housed in temperature-controlled animal holding facilities on slat flooring (Tenderfoot Flooring, Tandem Products, Minneapolis, MN) and given at least 21 ft2 of floor space as required by the Guide for the Care and Use of Laboratory Animals.11 A ration of alfalfa pellets (7060 Teklad Ruminant Diet, Harlan Laboratories, Indianapolis, IN) mixed with sweet feed (oats, corn, and molasses from Bombay, Kenyon, MN) in a 3:1 ratio was fed daily; this diet was supplemented with hay cubes and cut grass hay (locally sourced). Rooms were on a 13:11-h light cycle and were sanitized daily.
SRB administration.
The formulation concentration of SRB (SR Veterinary Technologies, Windsor, CO) was 10 mg/mL for injection in 5-mL vials, stored refrigerated at 38 °F (3.3 °C). Before injection, the solution was allowed to reach room temperature. The intended SRB dose was determined by calculating the total amount of conventional buprenorphine hydrochloride typically administered to sheep during a standard 3-d postoperative regimen (0.03 mg/kg every 8 h for 72 h). Thus, the total SRB dose was 0.27 mg/kg.
In all 4 sheep, SRB (0.27 mg/kg) was administered by a single intramuscular injection in the lumbosacral muscle. After a minimal washout period of 1 mo, SRB (0.27 mg/kg) was administered by a single subcutaneous injection in the neck or axilla region in the same 4 sheep.
Blood samples.
Blood (6 mL) was collected at 9 time points: baseline (0 min) and then at 30 and 60 min and at 6, 12, 18, 24, 48, and 72 h after both the intramuscular and subcutaneous injections. Blood was collected from the jugular vein by using an 18-gauge vacuum phlebotomy tube directly into a 10-mL EDTA tube and centrifuged within 2 h after collection to obtain plasma. Because buprenorphine was still detectable in plasma (with concentrations still increasing, in some sheep) at 72 h after the intramuscular injection, an additional 4 time points were monitored after subcutaneous injection only: days 4, 5, 6, and 7.
Plasma was stored at –80°C for a maximum of 1 wk and then shipped on dry ice to Protea Biosciences (Morgantown, WV) for determination of buprenorphine and norbuprenorphine plasma concentrations. The samples were processed by liquid–liquid extraction and analyzed by using a quantitative liquid chromatography–tandem mass spectrometry assay. Aliquots (1 mL) of plasma were extracted by using methanol plus a buprenorphine-D4 and norbuprenorphine-D3 primary internal standard. After centrifugation, the sample supernatants were transferred to a 96-well plate and dried under nitrogen at 50 °C for approximately 40 min, after which samples were reconstituted in 200 µL of 0.1% formic acid in water. The 96-well plate was sealed and vortexed for approximately 1 min. Samples were loaded for analysis into an Aquasil (C18, 50 × 2.1 mm, 5 µm; Thermo Scientific, Waltham, MA) analytical column for analysis by the API 4000 and Q-Trap 5500 (ABSciex, Toronto, Canada). The calibration curve ranged from 0.1 to 50 ng/mL for buprenorphine and 0.4 to 200 ng/mL for norbuprenorphine. This commercial quantitative assay is performed according to a standard operating procedure under an established quality system for human and animal samples.
Thermal withdrawal thresholds.
Thermal stimulation devices are used to assess the degree of nociception in large animal models.35 In the current study, a thermal portable device was used to assess SRB-induced antinociception in all 4 sheep. The heat source consisted of a tungsten halogen lamp powered by a 12-V battery. The intensity of the light emitted was 250,000 candela. A planoconvex lens, with a focal length of 120 mm, concentrated the light. The lens was held in place by a metal housing. The temperature at the focal spot was 90 °C. Sheep were acclimated to the presence of investigative staff and to the thermal portable device.
During thermal testing, the sheep were not restrained and remained in their familiar home cage. When typical behavior (for example, feeding, ruminating) and lack of interest in the device were present, focal heat was applied to the cutaneous trunci region and to the coronary band (lateral claw) bilaterally. The application of a nociceptive stimulus to the coronary band in horses and cutaneous trunci in pigs is a robust method to evaluate analgesic efficacy.5,35 The distance from the light emitting heat source was 12 in. to the target site, which formed an expected shape at the correct distance, so that the temperature was the same for each repetition and each animal. Measurement was repeated 3 times for each site, alternating between the cutaneous trunci, right leg, and left leg and then averaged. The rest time between sites occurred as the equipment was shifted quickly to the next site. By using a stopwatch, the time interval from the start of light impact until a response was measured; however, to prevent thermal injury, the thermal stimulus was halted after 40 s.
Note that, at the outset of this study before SRB treatment began, a measurement trial was performed in all 4 sheep both to train the single person that performed the testing throughout the experimental phase and verify that the assay provoked a typical withdrawal response to focal thermal exposure. The expected response was a panniculus response (twitch) of the cutaneous trunci region or a withdrawal response (lifting or flicking of the leg) at the coronary band. Lifting or flicking of the leg occurred consistently, but the panniculus response was inconsistent; for this reason, we halted the use of that region for thermal testing. The thermal stimulus was applied to the coronary band of the sheep, on both the right and left rear leg just prior to SRB administration and then at the 9 time points through 72 h, 3 times on each leg in an alternating fashion. The time to the withdrawal response was measured (in s) and averaged. An absent response at the cutoff time was recorded as 40 s.
Physical examination.
The 4 sheep underwent a physical examination to identify potential side effects commonly associated with opioid use (for example, sedation, respiratory depression, or gastrointestinal signs) at baseline (0 min); 30 and 60 min and at 6, 12, 18, 24, 48, and 72 h after both the intramuscular and subcutaneous injections; and on days 4, 5, 6, and 7 after subcutaneous administration only. Each examination included a general assessment (appetite, attitude, lethargy or ataxia, and urinary and fecal output and consistency) plus temperature, heart rate, respiratory rate, and any rumen sounds were also recorded. A heart rate of 70 to 80 beats per minute and a respiratory rate of 16 to 34 breaths per minute was considered typical.13 Injection sites were evaluated directly for possible reaction (swelling, erythema, pruritus). In addition, sites exposed to the focal thermal stimulus were examined carefully for any evidence of lesions due to thermal damage. Sheep were weighed before each injection and after the completion of follow-up, as an indirect assessment of appetite and general measure of health.
Statistical analysis.
PKSolver 2.0 was used to determine the pharmacokinetic parameters for both the intramuscular and subcutaneous injections and to generate noncompartmental methods: maximal plasma concentration, time of maximal plasma concentration, AUC0-t, mean residence time, and t1/2.43 Demographic data and nociceptive data (withdrawal from the thermal stimulus) are expressed as means ± 1 SD. Prism (GraphPad Software, San Diego, CA) was used for correlation analysis to estimate the relationship between measured buprenorphine concentrations and thermal withdrawal response time. The Spearman nonparametric correlation was used with a 2-tailed P value. A P value of less than 0.05 was considered significant.
Results
Safety.
The baseline physical examination confirmed the healthy status of all 4 sheep. They remained within normal limits for temperature, pulse, respiration, and rumination—with no indication of excessive sedation or opioid-induced depression of the respiratory or cardiovascular system—after treatment with SRB by both the intramuscular and subcutaneous route.13,42 All sheep maintained or gained weight during study period (3 to 7 d), suggesting no decrease in appetite. No adverse events (including muscle soreness, limping, wheals, pruritus, erythema, and other reactions) were observed.
Pharmacokinetics.
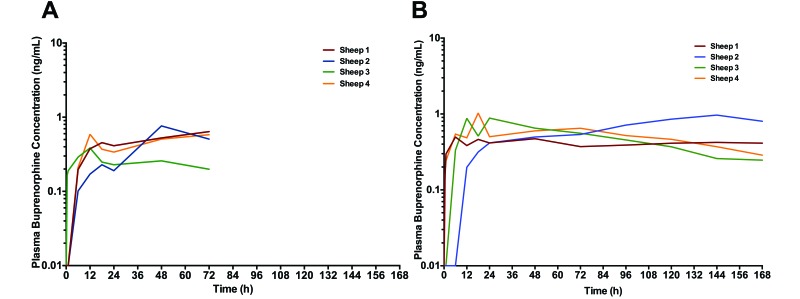
During the 12 h after both the intramuscular and subcutaneous injections, plasma buprenorphine concentrations steadily increased, by which time all 4 sheep reached the minimum therapeutic threshold (0.1 ng/mL) and maintained a steady state for at least 72 h (Figure 1). Yet the sheep generally required at least 48 h to achieve therapeutic plasma concentrations in the range recommended for major surgery (0.5 to 0.7 ng/mL).7 A single sheep (no. 3) failed to reach the threshold of 0.5 ng/mL and only after the intramuscular injection.
Figure 1.
Plasma buprenorphine concentrations in individual sheep after a single dose of sustained-release buprenorphine (0.27 mg/kg) administered (A) intramuscularly or (B) subcutaneously. Sheep were followed for 72 h after the intramuscular injection and 168 h after the subcutaneous injection.
Concentrations peaked between 12 and 72 h after the intramuscular injection (Table 1, Figure 1 A) and between 6 and 144 h after the subcutaneous injection (Table 1, Figure 1 B). No significant difference in the average maximal plasma buprenorphine concentration occurred between routes of administration (Table 1). After reaching peak plasma concentrations, the overall plasma concentrations remained above 0.2 ng/mL for the duration of follow-up in all 4 sheep, regardless of the route of administration (intramuscular or subcutaneous).
Table 1.
Selected pharmacokinetic values for sustained-release buprenorphine administered intramuscularly (n = 4) and subcutaneously (n = 4) to sheep
| Intramuscular |
Subcutaneous |
|||||||||
| Sheep |
Sheep |
|||||||||
| 1 | 2 | 3 | 4 | Mean ± 1 SD | Mean ± 1 SD | 1 | 2 | 3 | 4 | |
| Tmax (h) | 72 | 48 | 12 | 12 | 36 ± 64 | 48 ± 64 | 6 | 144 | 24 | 18 |
| Cmax (ng/mL) | 0.64 | 0.76 | 0.38 | 0.59 | 0.60 ± 0.20 | 0.80 ± 0.20 | 0.50 | 0.96 | 0.88 | 1.03 |
| AUC0–72 | 32.6 | 30.3 | 18.0 | 31.3 | 28.1 ± 6.8 | 36.7 ± 8.8 | 30.7 | 27.8 | 45.7 | 42.5 |
| MRT (h) | NE | NE | 148.7 | NE | ND | 122.8 ± 1.3 | NE | NE | 121.8 | 123.7 |
| t1/2 (h) | NE | NE | 102.0 | NE | ND | 72.9 ± 5.3 | NE | NE | 76.6 | 69.1 |
Cmax, maximal plasma concentration; MRT, mean residence time; ND, not determined because of insufficient data to determine the mean and SD; NE, not estimated, but concentrations in the terminal phase showed an ascending tendency; Tmax, time at peak plasma concentration.
Individual plasma concentrations were somewhat variable between animals, especially between higher-weight male and average-weight female sheep (Figure 1). When stratified by age, sex, or weight, none of the differences was statistically significant.
Overall, 72-h bioavailability was comparable between routes: 28.1 ± 6.8 ng/mL × h for the intramuscular period compared with 36.7 ± 8.8 ng/mL × h for the subcutaneous period. Thus, there was no evidence of immediate release of buprenorphine in any of the sheep.
Plasma concentrations of norbuprenorphine metabolite were measured in parallel with buprenorphine. Norbuprenorphine was undetectable.
Antinociception.
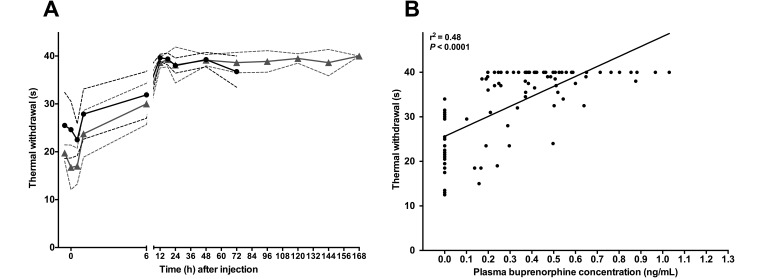
In all 4 sheep within 12 h after SRB administration by either route of administration, the withdrawal response to coronary band thermal stimulation was decreased from baseline (Figure 2 A). Specifically the relationship between the plasma buprenorphine concentration and thermal withdrawal time was statistically significant (P < 0.0001): as the concentrations increased, the thermal withdrawal time declined (r2 = 0.48, r = 0.69, 95% confidence interval = 0.57–0.78).
Figure 2.
(A) The relationship between plasma buprenorphine concentrations and withdrawal time from the thermal stimulus is shown before (‘pre’), at (time 0), and after a single dose (0.27 mg/kg) of sustained-release buprenorphine administered intramuscularly (black circles) or subcutaneously (gray triangles). Dashed lines indicate 1 SD from the mean. (B) According to our correlation analysis, the response to the thermal withdrawal stimulus declined as plasma buprenorphine concentrations increased.
Discussion
In this study, we found that the SRB dose of 0.27 mg/kg, given as a single intramuscular injection or a single subcutaneous injection, was a well-tolerated analgesic in healthy adult male and female sheep. Both routes yielded similar profiles (Table 1) suggesting that pharmacokinetics were not strongly influenced by the route of administration. Admittedly, the shorter length of follow-up after the intramuscular injection (72 h), as compared with the subcutaneous injection (168 h), could have biased our results. Average plasma concentrations peaked at 0.6 ng/mL after the intramuscular injection and at 0.8 ng/mL, after the subcutaneous injection. Those peak concentrations were substantially lower than the peak concentrations observed after injection of conventional buprenorphine.28 Interestingly, even at lower concentrations than the target therapeutic range recommended for moderate to severe pain (0.5–0.7 ng/mL),7,8,20 we found that SRB significantly sustained antinociceptive activity against a thermal stimulus in sheep, as compared with baseline, for at least 72 h A steady-state concentration was achieved with the depot formulation. This effect could confer an advantage over the immediate-release formulation, with which end-of-dose breakthroughs may occur because of its repeated-dosing ‘sawtooth’ profile. Failure to detect norbuprenorphine is likely attributable to concentrations being less than the assay's lower limit of detection (0.4 ng/mL).
The pilot sample size does not allow for an appropriate statistical interpretation of age, sex, or weight factors. The variability we observed suggests that demographic differences might influence pharmacokinetics. In particular, in higher-weight male sheep, the plasma concentration consistently showed an ascending tendency in the terminal elimination phase, indicating that body composition might affect the sustained-release formulation depot.
The intra- and interindividual plasma concentration variability that we observed in our 4 sheep is consistent with similar variability in large animals.29 However because we observed only a 2-fold difference in peak plasma concentrations between sheep (range, 0.38 to 0.76 ng/mL after intramuscular injection compared with 0.5 to 1.03 ng/mL after subcutaneous injection), individualized dose adjustments are unnecessary. Some variability can be expected between animals with different body types (that is, lean compared with obese): tissue differences in the muscle mass or fat surrounding the drug reservoir may affect the depot as well as the depth of injection. Given our finding that individual sheep responded at least somewhat differently to SRB, yielding a faster or slower initial onset of therapeutic effect, and in light of its pharmacokinetic profile, we recommend: 1) dosing at least 12 h (ideally 48 h) prior to painful procedures or providing additional coverage during the onset period (when the study permits) and 2) following a robust clinical evaluation program to monitor pain postoperatively.30,40
The planned type of procedure is relevant with regard to the severity and duration of pain. The dose selected for this study (0.27 mg/kg) was sufficient to reach minimal therapeutic concentrations in all sheep within 12 h, regardless of the route of administration. Likewise, thermal thresholds increased significantly between baseline and 12 h, and lack of response persisted for at least 72 h. For minor procedures (for example, subcutaneous incision mass removal or implantation, minor teeth extractions, biopsies, bone marrow aspiration), a plasma buprenorphine concentration of at least 0.1 ng/mL is considered therapeutic.29 But for major surgery (for example, thoracotomy, sternotomy, osteotomy, laparotomy), a plasma buprenorphine concentration of at least 0.5 ng/mL is recommended.8,42 Most of the sheep in our study achieved steady-state concentrations above 0.5 ng/mL, but doing so took as long as 48 h. Sheep may require an increased starting dose, or dual coverage with an additional analgesic, during the first 48 h after a procedure. That plasma buprenorphine concentrations in plasma persisted for periods of 1 wk (or more) requires special consideration in models where opioid-sensitive physiologic and immunologic parameters are under investigation.
To the best of our knowledge, this study is the first to use thermal nociception on the coronary band in sheep, although that technique is commonly used in large animals5,21,24,33 and has been used in lamb studies.10 Panniculus response (skin twitch) occurring in relation to the heat stimulus to the cutaneous trunci was not comparable to what has been observed in other models. The inconsistent results at this site, even in the same animal, and unacceptable variability between sheep could be related to the unique coat of characteristics in sheep. Given our observations, we cannot recommend the application of a focal thermal stimulus to the cutaneous trunci in sheep. In contrast, a ‘typical’ response to stimulus (obvious lifting or flicking of the leg) occurring in relation to the heat stimulus to the coronary band was comparable to what has been observed in other large animal models and in lambs in which this technique was used. The measurements performed by using the coronary band in triplicate bilaterally allowed us to average 6 measurements at each time point and across the group to characterize a representative response at each time point (Figure 2 A). Note that the noninvasive thermal nociception test might overestimate the analgesic effect and might not fully approximate the mechanical nociception relevant to surgical manipulation. Clearly, more mechanical nociception efficacy data are needed regarding SRB before we can recommend it for independent use in major surgery. In our study, we observed a moderate association between increasing buprenorphine plasma concentrations and withdrawal response to the thermal stimulus. The withdrawal response did not return to baseline during follow-up, perhaps because plasma buprenorphine concentrations persistently remained above 0.2 ng/mL. There is always a possibility for some degree of habituation in thermal nociceptive assays, an effect that we cannot rule out in the absence of a control group. However, in similar studies in large animals using matched controls absent analgesia, habituation was not observed during serial application of this assay technique.35
A multimodal analgesic approach—using a combination of opioids, NSAID, local pain blocks, and α2 agonists—can enhance analgesia, especially for major surgery.13-15 As we outline here, a 1:1 dose conversion is a reasonable starting point when substituting SRB in place of conventional buprenorphine in these analgesic regimens. A randomized blinded dose–response study, ideally in combination with relevant experimental manipulation (for example, surgical, orthopedic) and conventional controls, is necessary to firmly establish a recommended range for various applications.
In conclusion, in our pilot study of 4 sheep, we found that SRB was well-tolerated and achieved steady plasma concentrations for long periods, with analgesic efficacy in a contrived thermal stimulus assay. This formulation could be a promising alternative to repeated buprenorphine dosing in sheep. A long-acting analgesic not only provides the important advantage of limiting the chance of end-of-dose break through pain but also minimizes the number of times that sheep must be separated and restrained, because only a single injection is needed. Injectable SRB should be strongly considered as a practical component of a multimodal analgesic approach designed to minimize pain and distress in sheep used for research.
Acknowledgments
We thank Research Animal Resources for housing the sheep used for this study and for technical assistance. We thank Jack Risdahl for providing the portable thermal testing device. We thank Experimental Surgical Services for providing healthy sheep. We thank William Lance and Steve Kirschner (Wildlifeaceuticals) for providing SRB and for performing LC–MS/MS at no cost to us. We thank Mary Knatterud for editorial assistance.
References
- 1.Ahern BJ, Soma LR, Boston RC, Schaer TP. 2009. Comparison of the analgesic properties of transdermally administered fentanyl and intramuscularly administered buprenorphine during and following experimental orthopedic surgery in sheep. Am J Vet Res 70:418–422. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan-Smith HM, Rennie AD, Vitale A, Pollo S, Prescott MJ, Morton DB. 2005. Harmonising the definition of refinement. Anim Welf 14:379–384. [Google Scholar]
- 3.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 4.Catbagan DL, Quimby JM, Mama KR, Rychel JK, Mich PM. 2011. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 72:461–466. [DOI] [PubMed] [Google Scholar]
- 5.Chambers JP, Livingston A, Waterman AE. 1990. A device for testing nociceptive thresholds in horses. Vet Anaesth Analg 17:42–44. [Google Scholar]
- 6.Cowan A, Lewis J, Macfarlane I. 1977. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans HC, Easthope SE. 2003. Transdermal buprenorphine. Drugs 63:1999–2010. [DOI] [PubMed] [Google Scholar]
- 8.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 9.Gallegos RP, Nockel PJ, Rivard AL, Bianco RW. 2005. The current state of in vivo preclinical animal models for heart valve evaluation. J Heart Valve Dis 14:423–432. [PubMed] [Google Scholar]
- 10.Guesgen MJ, Beausoleil NJ, Minot EO, Stewart M, Jones G, Stafford KJ. 2011. The effects of age and sex on pain sensitivity in young lambs. Appl Anim Behav Sci 135:51–56. [Google Scholar]
- 11.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 12.Johnson RE, Fudala PJ, Payne R. 2005. Buprenorphine: considerations for pain management. J Pain Symptom Manage 29:297–326. [DOI] [PubMed] [Google Scholar]
- 13.Kahn CM, Line S. 2010. The Merck veterinary manual. [Google Scholar]
- 14.Kehlet H, Dahl JB. 1993. The value of ‘multimodal’ or ‘balanced’ analgesia in postoperative pain treatment. Anesth Analg 77:1048–1056. [DOI] [PubMed] [Google Scholar]
- 15.Kehlet H, Wilmore DW. 2002. Multimodal strategies to improve surgical outcome. Am J Surg 183:630–641. [DOI] [PubMed] [Google Scholar]
- 16.Kim T, Kim J, Kim S. 1993. Extended-release formulation of morphine for subcutaneous administration. Cancer Chemother Pharmacol 33:187–190. [DOI] [PubMed] [Google Scholar]
- 17.Levy MH. 2001. Advancement of opioid analgesia with controlled-release oxycodone. Eur J Pain 5:113–116. [DOI] [PubMed] [Google Scholar]
- 18.Levy RJ, Vyavahare N, Ogle M, Ashworth P, Bianco R, Schoen FJ. 2003. Inhibition of cusp and aortic wall calcification in ethanol- and aluminum-treated bioprosthetic heart valves in sheep: background, mechanisms, and synergism. J Heart Valve Dis 12:209–216, discussion 216. [PubMed] [Google Scholar]
- 19.Lindhardt K, Ravn C, Gizurarson S, Bechgaard E. 2000. Intranasal absorption of buprenorphine—in vivo bioavailability study in sheep. Int J Pharm 205:159–163. [DOI] [PubMed] [Google Scholar]
- 20.Livingston A, Nolan A, Waterman A. 1986. The relationship between analgesia induced by and plasma concentration of buprenorphine in the sheep. Br J Pharmacol 88:363P.2425878 [Google Scholar]
- 21.Luna SP, Lopes C, Rosa A, Oliveira F, Crosignani N, Taylor P, Pantoja J. 2014. Validation of mechanical, electrical, and thermal nociceptive stimulation methods in horses. Equine Vet J 47:609–614. [DOI] [PubMed] [Google Scholar]
- 22.Martini L, Fini M, Giavaresi G, Giardino R. 2001. Sheep model in orthopedic research: a literature review. Comp Med 51:292–299. [PubMed] [Google Scholar]
- 23.Meeusen EN, Snibson KJ, Hirst SJ, Bischof RJ. 2009. Sheep as a model species for the study and treatment of human asthma and other respiratory diseases. Drug Discov Today Dis Models 6:101–106. [Google Scholar]
- 24.Mohling CM, Johnson AK, Abell C, Stalder KJ, Karriker LA, Coetzee JF, Millman ST. 2014. Thermal and mechanical nociception threshold tests as objective tools to measure painful and nonpainful lameness phases in multiparous sows. Animal Industry Report. 660. [Cited 15 October 2014] Available at: http://lib.dr.iastate.edu/ans_air/vol660/iss1/78. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama S, Sibley L, Gunther RA, Holcroft JW, Kramer GC. 1984. Small-volume resuscitation with hypertonic saline (2400 mOsm/liter) during hemorrhagic shock. Circ Shock 13:149–159. [PubMed] [Google Scholar]
- 26.Newman E, Turner A, Wark J. 1995. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone 16: 277S–284S. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson B. 2009. Benefits of extended-release opioid analgesic formulations in the treatment of chronic pain. Pain Pract 9:71–81. [DOI] [PubMed] [Google Scholar]
- 28.Nolan A, Livingston A, Waterman A. 1987. Investigation of the antinociceptive activity of buprenorphine in sheep. Br J Pharmacol 92:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56. [PMC free article] [PubMed] [Google Scholar]
- 30.Pajor EA, Marchant-Forde JN. 2011. Scientific evaluation of behavior, welfare, and enrichment. Proceedings of the 45th Congress of the International Society for Applied Ethology. 31 July–4 August 2011, Indianapolis, Indiana. [Google Scholar]
- 31.Pergolizzi J, Aloisi AM, Dahan A, Filitz J, Langford R, Likar R, Mercadante S, Morlion B, Raffa RB, Sabatowski R. 2010. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract 10:428–450. [DOI] [PubMed] [Google Scholar]
- 32.Plumb DC. 2011. Plumb's veterinary drug handbook. Hoboken (NJ): Wiley–Blackwell. [Google Scholar]
- 33.Poller C, Hopster K, Rohn K, Kastner SB. 2013. Evaluation of contact heat thermal threshold testing for standardized assessment of cutaneous nociception in horses—comparison of different locations and environmental conditions. BMC Vet Res 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinn RW. 2013. Animal models for bench to bedside translation of regenerative cardiac constructs. Prog Pediatr Cardiol 35:91–94. [Google Scholar]
- 35.Risdahl JM, Chao C, Murtaugh MP, Peterson PK, Molitor TW. 1992. Acute and chronic morphine administration in swine. Pharmacol Biochem Behav 43:799–806. [DOI] [PubMed] [Google Scholar]
- 36.Rivard AL, Suwan PT, Imaninaini K, Gallegos RP, Bianco RW. 2007. Development of a sheep model of atrial fibrillation for preclinical prosthetic valve testing. J Heart Valve Dis 16:314–323. [PubMed] [Google Scholar]
- 37.Russell WMS, Burch RL, Hume CW. 1959. The principles of humane experimental technique. London (United Kingdom): Methuen. [Google Scholar]
- 38.Scheerlinck JP, Snibson KJ, Bowles VM, Sutton P. 2008. Biomedical applications of sheep models: From asthma to vaccines. Trends Biotechnol 26:259–266. [DOI] [PubMed] [Google Scholar]
- 39.Schoen FJ, Hirsch D, Bianco RW, Levy RJ. 1994. Onset and progression of calcification in porcine aortic bioprosthetic valves implanted as orthotopic mitral valve replacements in juvenile sheep. J Thorac Cardiovasc Surg 108:880–887. [PubMed] [Google Scholar]
- 40.Unruh AM. 1996. Gender variations in clinical pain experience. Pain 65:123–167. [DOI] [PubMed] [Google Scholar]
- 41.Viscusi ER, Martin G, Hartrick CT, Singla N, Manvelian G, EREM Study Group 2005. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology 102:1014–1022. [DOI] [PubMed] [Google Scholar]
- 42.Waterman AE, Livingston A, Amin A. 1991. Further studies on the antinociceptive activity and respiratory effects of buprenorphine in sheep. J Vet Pharmacol Ther 14:230–234. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Huo M, Zhou J, Xie S. 2010. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–314. [DOI] [PubMed] [Google Scholar]