Abstract
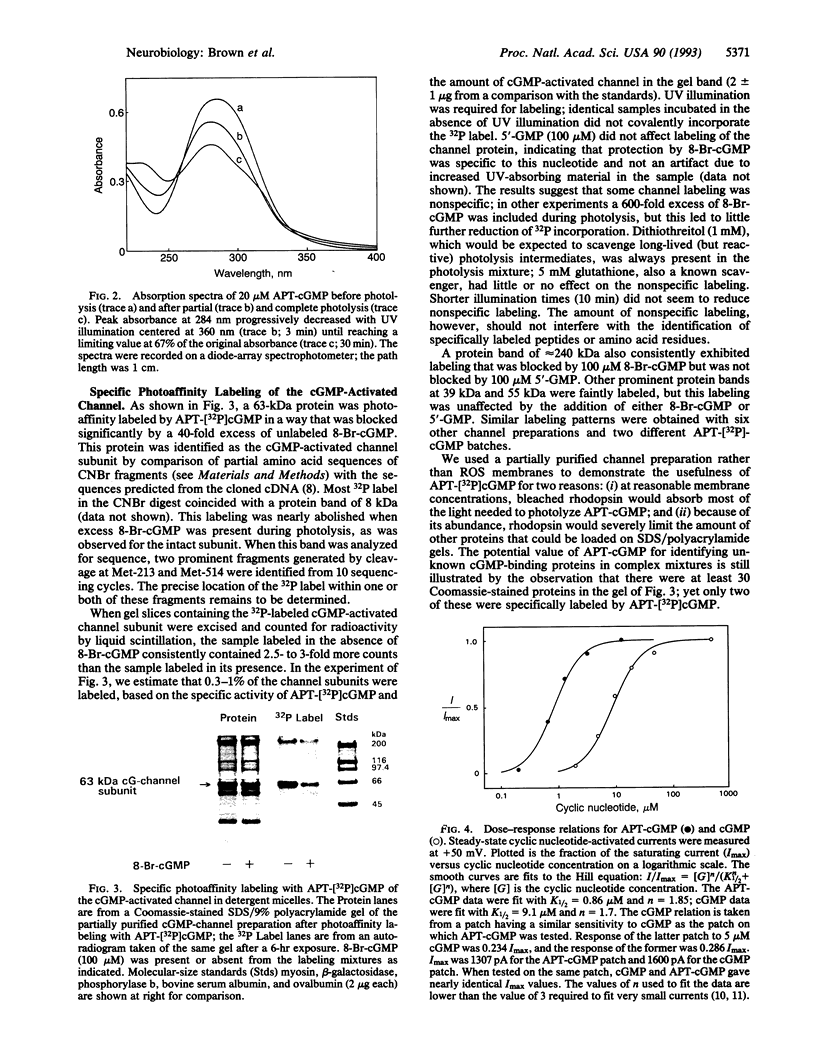
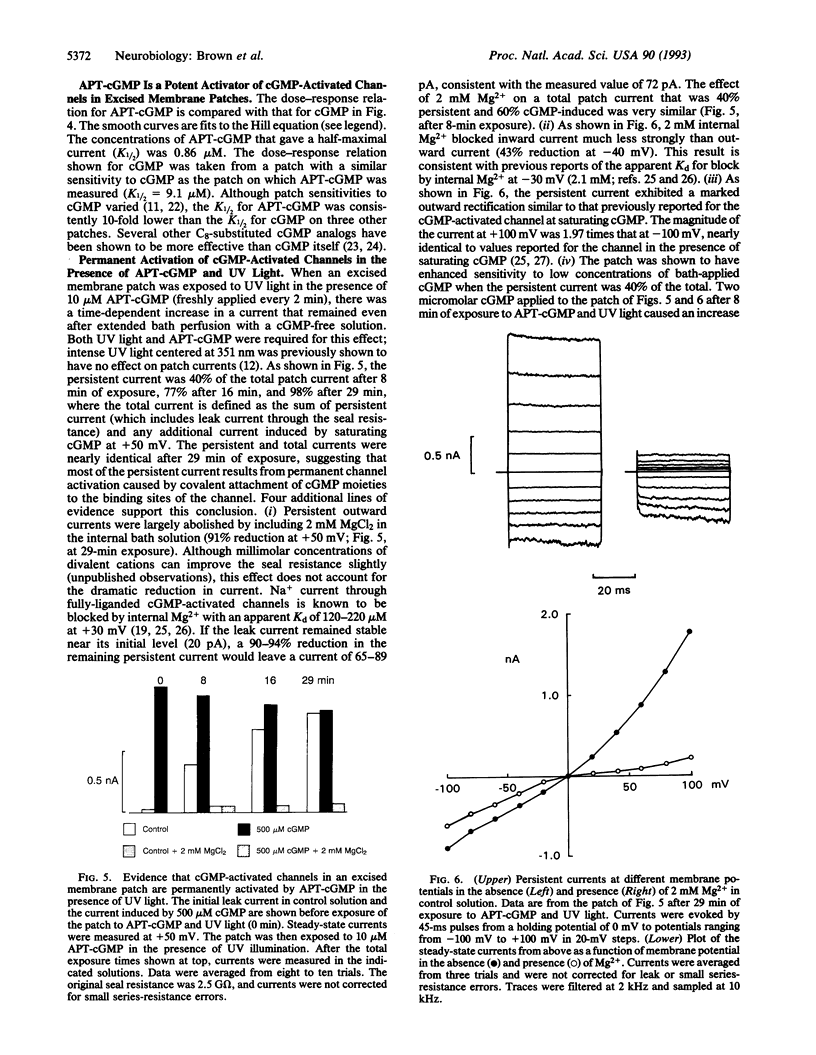
Cyclic nucleotide-gated ion channels play a key role in visual excitation and a variety of other signaling pathways. The photoaffinity probe 8-p-azidophenacylthio-cGMP (APT-cGMP) has been developed for structural and functional studies of the cGMP-activated channel of retinal rod outer segments. Using this analog, we have demonstrated both specific labeling of the channel in a partially purified biochemical preparation from bovine rod outer segments and permanent activation of the channel current in excised membrane patches from salamander outer segments. After UV illumination, a 32P-labeled version of APT-cGMP was shown by SDS/PAGE and autoradiography to be covalently attached to the 63-kDa channel subunit. This incorporation was significantly reduced by 8-Br-cGMP but was not reduced by 5'-GMP. In patch-clamp experiments APT-cGMP was a potent activator of the channel; APT-cGMP typically opened half of the channels in a patch at a 10-fold lower concentration than cGMP. Exposure of membrane patches to UV light in the presence of APT-cGMP resulted in a persistent current observed in the absence of bath-applied nucleotide. This current increased with repeated exposure of the patch to both UV light and fresh APT-cGMP, approaching the maximum current originally evoked by saturating (500 microM) cGMP. At this point, addition of 500 microM cGMP caused a negligible increase in current. The persistent current had several other properties expected of current through cGMP-activated channels: it was outwardly rectifying; outward current was blocked > 90% by 2 mM internal Mg2+, whereas inward current was blocked much less efficiently; a low concentration of cGMP caused a larger increase in current atop a half-maximal persistent current than it did originally. We conclude that the persistent current was caused by the covalent tethering of cGMP moieties to channel binding sites, resulting in irreversible channel activation. APT-cGMP should prove useful for further studies of these and similar cGMP-binding sites and in the identification of unknown cGMP-binding proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenhofen W., Ludwig J., Eismann E., Kraus W., Bönigk W., Kaupp U. B. Control of ligand specificity in cyclic nucleotide-gated channels from rod photoreceptors and olfactory epithelium. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9868–9872. doi: 10.1073/pnas.88.21.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A., Sorbi R. T. Binding stoichiometry of a fluorescent cGMP analogue to membranes of retinal rod outer segments. Eur J Biochem. 1985 Nov 15;153(1):49–53. doi: 10.1111/j.1432-1033.1985.tb09265.x. [DOI] [PubMed] [Google Scholar]
- Cavaggioni A., Sorbi R. T. Cyclic GMP releases calcium from disc membranes of vertebrate photoreceptors. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3964–3968. doi: 10.1073/pnas.78.6.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamartino G., Menini A., Torre V. Blockage and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J Physiol. 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. J., Hanke W., Kaupp U. B. Identification, purification, and functional reconstitution of the cyclic GMP-dependent channel from rod photoreceptors. Proc Natl Acad Sci U S A. 1987 Jan;84(2):585–589. doi: 10.1073/pnas.84.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Henderson D. A cyclic GMP-activated channel in dissociated cells of the chick pineal gland. Nature. 1991 Oct 24;353(6346):756–758. doi: 10.1038/353756a0. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Geahlen R. L., Haley B. E., Krebs E. G. Synthesis and use of 8-azidoguanosine 3',5'-cyclic monophosphate as photoaffinity label for cyclic GMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1979 May;76(5):2213–2217. doi: 10.1073/pnas.76.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. E., Brautigan D. L., Zimmerman A. L. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992 Oct;9(4):739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L., Yau K. W. Cyclic GMP-sensitive conductance in outer segment membrane of catfish cones. Nature. 1985 Sep 5;317(6032):61–64. doi: 10.1038/317061a0. [DOI] [PubMed] [Google Scholar]
- Hixson S. H., Hixson S. S. P-Azidophenacyl bromide, a versatile photolabile bifunctional reagent. Reaction with glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Sep 23;14(19):4251–4254. doi: 10.1021/bi00690a016. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Brown R. L., Stryer L., Baylor D. A. Interactions between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods. J Gen Physiol. 1993 Jan;101(1):1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Kolesnikov S. S., Rebrik T. I., Zhainazarov A. B., Tavartkiladze G. A., Kalamkarov G. R. A cyclic-AMP-gated conductance in cochlear hair cells. FEBS Lett. 1991 Sep 23;290(1-2):167–170. doi: 10.1016/0014-5793(91)81251-3. [DOI] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C., Levin A., Branton D. Copper staining: a five-minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Nov 1;166(2):308–312. doi: 10.1016/0003-2697(87)90579-3. [DOI] [PubMed] [Google Scholar]
- Marunaka Y., Ohara A., Matsumoto P., Eaton D. C. Cyclic GMP-activated channel activity in renal epithelial cells (A6). Biochim Biophys Acta. 1991 Nov 18;1070(1):152–156. doi: 10.1016/0005-2736(91)90157-4. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Boswell K. H., Muneyama K., Simon L. N., Robins R. K., Shuman D. A. Synthesis and biochemical studies of various 8-substituted derivatives of guanosine 3',5'-cyclic phosphate, inosine 3',5'-cyclic phosphate, and xanthosine 3',5'-cyclic phosphate. Biochemistry. 1973 Dec 18;12(26):5310–5319. doi: 10.1021/bi00750a014. [DOI] [PubMed] [Google Scholar]
- Molday L. L., Cook N. J., Kaupp U. B., Molday R. S. The cGMP-gated cation channel of bovine rod photoreceptor cells is associated with a 240-kDa protein exhibiting immunochemical cross-reactivity with spectrin. J Biol Chem. 1990 Oct 25;265(30):18690–18695. [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Shinozawa T., Sokabe M., Terada S., Matsusaka H., Yoshizawa T. Detection of cyclic GMP binding protein and ion channel activity in frog rod outer segments. J Biochem. 1987 Aug;102(2):281–290. doi: 10.1093/oxfordjournals.jbchem.a122052. [DOI] [PubMed] [Google Scholar]
- Shinozawa T., Terada S., Matsusaka H., Yamashita S. Detection of Ca2+-dependent cyclic GMP binding protein in frog rod outer segments. FEBS Lett. 1987 Jul 27;219(2):293–295. doi: 10.1016/0014-5793(87)80238-7. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Thompson D. A., Khorana H. G. Guanosine 3',5'-cyclic nucleotide binding proteins of bovine retina identified by photoaffinity labeling. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2201–2205. doi: 10.1073/pnas.87.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol. 1992 Apr;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]



