Abstract
International consensus guidelines for the management of intraductal papillary mucinous neoplasm (IPMN) of the pancreas revised in 2012 (Fukuoka consensus) seem to be accepted well worldwide. Division of various factors to predict malignant transformation into two categories, i.e., “high-risk stigmata” and “worrisome features”, is also accepted as practically useful for stratifying the risk factors. Our current interest resides in the development of noninvasive and/or invasive pancreatic cancer in areas of the pancreas distinct from IPMN. Invasive pancreatic cancers derived from and concomitant with IPMN should be distinguished to clarify the incidence of each entity, although some more definitive method for differentiation has to be devised in some cases where histological distinction is obscure. IPMN is a clue to early detection of pancreatic cancer. The optimal surveillance protocol for IPMN on observation should be determined in consideration of both of these different pancreatic cancers.
Keywords: Intraductal papillary mucinous neoplasm (IPMN), main duct type, branch duct type, pancreatic cancer
Introduction
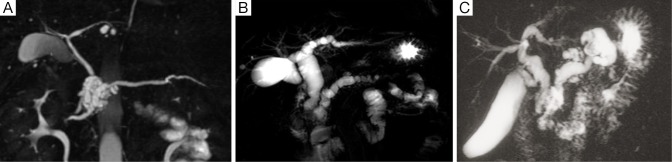
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a fascinating entity caused by proliferation of mucin-producing neoplastic epithelia and characterized by cystic or saccular dilation of the branch duct (BD-IPMN) and/or main duct (MD-IPMN) (1). IPMN with macroscopic features of both BD-IPMN and MD-IPMN is called mixed type at present (Figure 1A-C). An orifice of the duodenal papilla may be dilated with protruding mucin and present with “fish mouth appearance” in any type of IPMN but not in all cases (Figure 2). This unique endoscopic feature originally drew attention in Japan and led to the emergence of a new clinical entity “mucin-producing tumors of the pancreas” (2,3).
Figure 1.
Magnetic resonance cholangiopancreatograms showing macroscopic types of IPMN. (A) Branch duct type; (B) main duct type; (C) mixed type.
Figure 2.
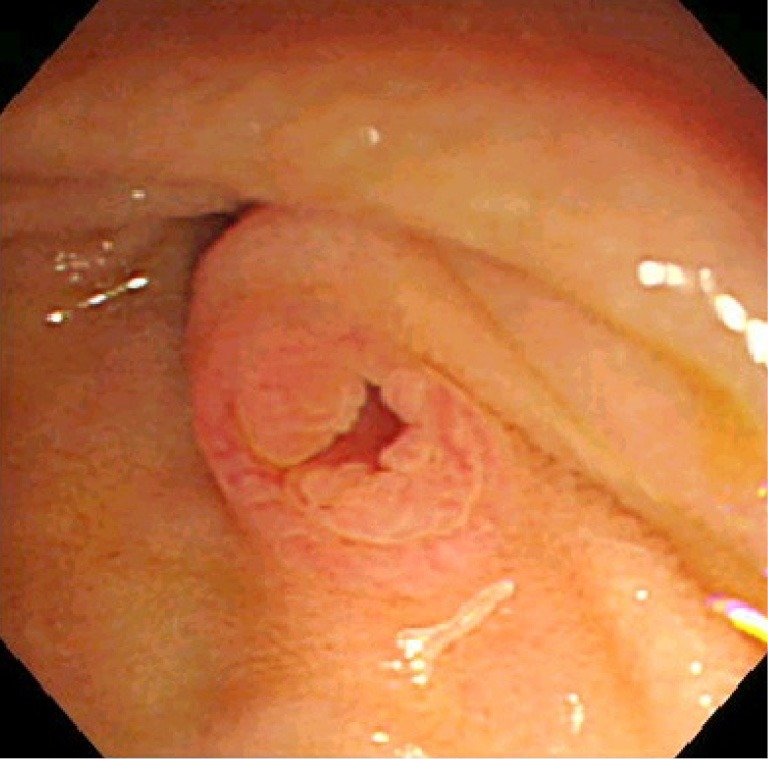
Endoscopic photograph of the patulous duodenal papilla dilated by mucin.
The clinical definition and management of IPMN were rather confused for a long time until the international consensus guidelines were issued by the International Association of Pancreatology in 2006 and revised in 2012 (1,4). These guidelines were widely used for consideration of surgical resection and observation. This review addresses the most important aspects of IPMN, i.e., association of pancreatic cancer and the consensus on the management of IPMN.
Malignant change in IPMN
IPMN is characterized by malignant transformation from low-grade dysplasia to high-grade dysplasia (HGD) and further to invasive carcinoma following adenoma-carcinoma sequence (5). MD-IPMN is more frequently associated with this malignant transformation than is BD-IPMN (4), necessitating surgical resection in more than a half of the patients, while most patients with BD-IPMN can be observed for a long time after the diagnosis. Overall, the prognosis after resection is generally favorable as long as its invasion remains within minimally invasive or in T1a status (the depth of stromal invasion <5 mm) (6).
As the definitive diagnosis of the malignant change is practically difficult, the presence of malignancy has to be predicted by combination of physical and imaging findings. Accumulation of evidences yielded the Sendai consensus for prediction of malignancy and the clinical management of IPMN in 2006 (4). MD-IPMN with dilation of the main pancreatic duct (MPD) >10 mm is frequently malignant and thus definitely a surgical indication. Regarding BD-IPMN, the criteria for resection consisting of clinical symptoms, positive cytology, presence of mural nodules, dilation of the MPD >6 mm, and cyst size >3 cm were accepted well and called the “Sendai criteria”. Of them, the presence of mural nodules most accurately demonstrated by endoscopic ultrasonography (EUS) is most reliable to predict malignancy (7-9). Ohno et al. (10) further categorized mural nodules into 4 types, i.e., low papillary, polypoid, papillary, and invasive. Of these, the papillary type and invasive type were claimed as most likely indicative of malignancy. On the other hand, the size of the mural nodule to predict malignancy has been various, being 5 mm (11,12), 7 mm (13), or 10 mm (14). The size of mural nodules to predict malignancy needs to be evaluated further.
Although the cyst size >3 cm was not claimed as an absolute indication for resection in the Sendai guidelines, many patients have been recommended surgery employing this criterion with relatively low rates of malignancy in surgical specimens, only 13-23% (15,16). On the contrary, there have been a few reports of invasive carcinoma found in BD-IPMNs ≤3 cm without mural nodules (17,18), although whether HGD should be included in malignancy or not remains undetermined. The relationship of the risk of malignancy to the cyst size should be evaluated independently of the effect of mural nodules or MPD dilation. Sadakari et al. (15) reported the frequency of malignancy of only 3.6% in BD-IPMNs ≥30 mm without mural nodules or MPD dilation (<5 mm), whereas it was 26.3% when the MPD diameter was ≥5 mm or more. Fritz et al. (19) reported that 17 of 69 patients (24.6%) with BD-IPMNs <3 cm showed malignant histological features (invasive carcinoma or carcinoma in situ), but EUS was not performed in all of their patients. In this regard, Wong et al. (17) confirmed the absence of Sendai criteria by EUS in 105 patients with BD-IPMN surgically resected. Twenty-four (34%) of 70 cysts ≤3 cm patients had invasive cancer, including 1 of 7 cysts (14%) <1 cm, 2 of 19 cysts (11%) 1-2 cm, and 21of 44 cysts (48%) 2-3 cm, while 15 of 35 cysts (43%) >3 cm had invasive cancer. Sixteen cysts <3 cm (23%) had HGD, including 3 of 7 cysts (43%) <1 cm, 3 of 19 cysts (16%) 1-2 cm, and 10 of 44 cysts (23%) 2-3 cm. Likewise, Shimizu et al. (13) analyzed 160 patients with malignant IPMN (noninvasive 100, invasive 60) who underwent EUS and claimed that 9.4% of them had no mural nodules. Koshita et al. (20) also reported that 9 of 21 patients with invasive cancer derived from IPMN had no mural nodules even on EUS. Therefore, the guidelines needed to be revised in 2012 (Fukuoka consensus), where the size criterion has been excluded from high-risk stigmata and moved down to the next category worrisome features to require thorough EUS examination (Table 1) (1). Goh et al. (21) reported that the high-risk stigmata of the Fukuoka consensus guidelines provided higher positive and negative values to predict high-risk IPMN than the Sendai consensus, 88% vs. 67% and 92.5% vs. 88%, respectively.
Table 1. Predictors of malignancy in branch duct IPMN.
| Category | Sendai consensus | Fukuoka consensus |
|---|---|---|
| High-risk stigmata | Presence of mural nodules; MPD >6 mm; symptoms; positive cytology (cyst size >3 cm)§ | Enhanced mural nodules; MPD ≥10 mm; jaundice associated with a cystic mass in pancreatic head |
| Worrisome features | Cyst size ≥3 cm; thickened enhanced cyst walls; MPD 6-9 mm; non-enhanced mural nodules; MPD stenosis with distal pancreatic atrophy; adjacent lymphadenopathy |
§, cyst size >3 cm is not an absolute indicator of malignancy until more evidences are available. IPMN, intraductal papillary mucinous neoplasm; MPD, main pancreatic duct.
Some other strategies may be more effective to predict malignancy in IPMN but more complicated. Kang et al. (22) reported that a greater rate (4.1 mm/year for malignant vs. 1.0 mm/year for benign; P=0.001) of cyst growth may be of additive value to predict malignant IPMN. Shimizu et al. (23) proposed a nomogram comprising multiple factors to raise the sensitivity of predicting malignancy. Ohtsuka et al. (24) indicated that an increase in the number of predictive factors in the Sendai consensus raised likelihood of malignancy.
Cytology of the pancreatic juice collected during ERCP or cyst fluid obtained by EUS-guided fine needle aspiration (FNA) is definitely the most reliable predictor of malignancy in IPMN. However, there are disadvantages inherent to ERCP and EUS-FNA, including complications associated with these endoscopic procedures (25,26), difficulty in cytological interpretation of obtained samples, and relatively low sensitivity even with enthusiastic potential improvements (27-33). Duodenal fluid may be a right choice to explore more safe and effective prediction of malignant IPMNs provided more sensitive biomarkers are identified in the future (34-37).
Pancreatic cancer distinct from IPMN
Another unique feature of IPMN recognized 17 years after the first recognition of this fascinating entity is the association of pancreatic ductal adenocarcinoma (PDAC) concomitant with but distinct from IPMN (38,39). Since Tanaka et al. (38) reported the first case of carcinoma in situ concomitant with a small benign BD-IPMN in 1997, synchronous and/or metachronous association of noninvasive and invasive ordinary pancreatic cancer continues to be reported mainly in Japan (40-45). As IPMN is very easy to detect by various imaging modalities such as ultrasonography, computed tomography, and magnetic resonance imaging, IPMN has become a definite target for early detection of sporadic pancreatic cancer (46,47). Although the frequency of concomitant pancreatic cancer in patients with IPMN is not very high, being 2.5% to 9.2% (48,49), the prevalence of IPMN is relatively high as reported to be 9.4% in 341 patients undergoing EUS for non-pancreatic indications (50). This means that the increased awareness of this phenomenon should lead to early detection of pancreatic cancer.
The incidence of distinct pancreatic cancer in patients with IPMN was evaluated in many retrospective studies (40,41,43,45,51-56). Tada et al. (40) discovered 5 PDACs (2.5%, 0.68% per year) in 197 patients with cystic pancreatic lesions, including 80 IPMNs and 117 “non-IPMN cysts” during an average of 3.8 years. Uehara et al. (41) conducted a prospective study on 60 patients with BD-IPMN <10 mm on US for a mean period of 87 months. PDAC developed in a part distinct from IPMN in 5 of them (8%), thus the 5-year rate 6.9% and yearly incidence 1.1%. On the contrary, malignant transformation was noted only in 2 of 60 patients with IPMNs (3%). Tanno et al. (51) found 4 patients with PDAC (7.2 per 1,000 patient-years) in 89 patients with BD-IPMN followed up for a median of 64 months (range, 25-158 months). The same group found synchronous or metachronous PDAC in 9 of 168 patients (5.4%) with BD-IPMN (43). There was statistically significant tendency toward the occurrence of PDAC in patients with the older age ≥70 years, female gender, smaller cyst size and MPD diameter.
A large-scale retrospective collective study by the Japan Pancreas Society showed distinct PDAC in 7 of 349 patients (2.0%) with BD-IPMN during a median follow-up period of 3.7 years, thus the yearly incidence 0.41% (45). On the other hand, 62 patients (17.8%) displayed progression of index IPMN. Most recently, Lafemina et al. (57) also reported a retrospective analysis of 170 patients with BD-IPMN with a median follow-up of 40 months. Of 97 patients who underwent resection, 79 had noninvasive IPMN and 18 “invasive carcinoma”. Of note is the fact that 5 of the 18 patients with invasive carcinoma developed PDAC in a region distinct from monitored IPMN (5.2%).
The diagnosis of concomitant PDAC is quite a new problem in the management of IPMN. PDAC may be overlooked even in cross-sectional images regularly obtained at 6-month intervals (58). Ingkakul et al. (42) reported elevated serum CA19-9 levels and worsening diabetes as significant predictors of 22 concomitant PDACs (9.3%) in 236 patients with BD-IPMN. Kanno et al. (59) also reported abnormal serum CA19-9 levels as a predictor of 7 PDACs concomitant with BD-IPMN in 159 patients.
Contrast enhanced CT, MRI, and EUS are usually employed for detection of PDAC concomitant with IPMN. Sakamoto et al. (60) reported a patient with a 10-mm PDAC found by EUS in the pancreatic tail distinct from a BD-IPMN in the pancreatic body. The PDAC was vaguely visualized by EUS and clearly delineated by contrast-enhanced harmonic EUS. The same group eventually reported 17 invasive IPMN and 11 concomitant PDACs in 167 patients with BD-IPMN (61). Noteworthy is that they further surveyed 102 patients whose BD-IPMNs had no high-risk stigmata or worrisome features by semiannual EUS and annual US, CT, and MRI. They found distinct PDAC in 7 patients, while no single patient showed invasive progression of monitored IPMN during surveillance for a median of 42 months, thus the 3- and 5-year rates of concomitant PDAC 4.0 % and 8.8 %, respectively. Only 3 of the 7 PDACs (43%) were visualized by CT or MRI even after detection by EUS. US could not detect any of the 7 PDACs. EUS seems to be essential for early detection of PDAC concomitant with IPMN. Ohtsuka et al. (56) further emphasized the significance of ERCP cytology for very early detection of PDAC in patients with IPMN.
The precise incidence of distinct PDAC arising in patients with IPMN and roles of diagnostic modalities in the early detection are to be determined by a large-scale prospective surveillance currently under way by the Japan Pancreas Society.
Fukuoka consensus
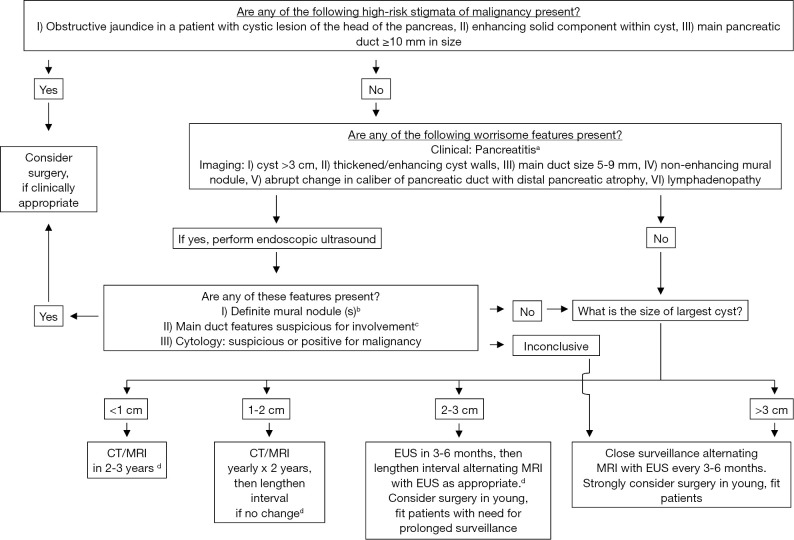
The two major changes in clinical management of IPMN reached in the Fukuoka consensus are a lowered threshold (≥5 mm) of the size of the MPD to increase the sensitivity of the diagnosis of MD-IPMN, and the introduction of two-layer criteria to predict malignancy in IPMN, i.e., “high-risk stigmata” to recommend immediate resection in all fit patients and “worrisome features” to warrant thorough examinations by EUS (Figure 3) (1). This revision is now widely accepted with higher sensitivity of the diagnosis of IPMN and prediction of malignancy (9,21), although the adequacy of the cyst size moved from the “high-risk stigmata” to “worrisome features” is still giving rise to much controversy (8,9,17,19). One meta-analysis declaimed that the cyst size >3 cm was associated most strongly with malignant IPMN (8), whereas another meta-analysis published later insisted that the presence of mural nodules should be regarded most highly suspicious of malignancy (9).
Figure 3.
Management algorithm with two-layer criteria to stratify risk factors to predict malignancy. Cited and reproduced with permission from Pancreatology 2012;12:183-197. a, pancreatitis may be an indication for surgery for relief of symptoms. b, differential diagnosis includes mucin. Mucin can move with change in patient position, may be dislodged on cyst lavage and does not have Doppler flow. Features of true tumor nodule include lack of mobility, presence of Doppler flow and FNA of nodule showing tumor tissue. c, presence of any one of thickened walls, intraductal mucin or mural nodules is suggestive of main duct involvement. In their absence main duct involvement is inconclusive. d, studies from Japan suggest that on follow-up of subjects with suspected BD-IPMN there is increased incidence of pancreatic ductal adenocarcinoma unrelated to malignant transformation of the BD-IPMN(s) being followed. However, it is unclear if imaging surveillance can detect early ductal adenocarcinoma, and, if so, at what interval surveillance imaging should be performed.
The Fukuoka consensus on pathological analyses of resected specimens of IPMN is that noninvasive carcinoma should be called HGD. As mentioned before, whether HGD should be operated on or can be observed remains unknown, because the natural history of IPMN progression after malignant transformation or the length of the period for HGD to become invasive carcinoma is not known. Although our previous study demonstrated that both HGD and T1a carcinoma (depth of invasion <5 mm) of IPMN (formerly called minimal invasion) are associated with a 100% survival rate after resection, it would be generally justifiable for most investigators to want to include HGD into the surgical indication, because the T1a carcinomas may already accompany lymph node metastasis in 20% (6).
Our particular interest resides in the appropriate methodology and time intervals for surveillance of BD-IPMN to check the malignant changes and development of distinct PDAC. The Fukuoka consensus advocates yearly follow-up if lesion is <10 mm in size, 6-12 monthly follow-up for lesions between 10 and 20 mm, and 3-6 monthly follow-up for lesions >20 mm as current reasonable approaches to surveillance, although the appropriate intervals between follow-up examinations remain to be determined. The Fukuoka consensus also recommends lengthening of the surveillance interval after 2 years of no change on images as did the Sendai guidelines. On the other hand, however, the Fukuoka consensus proposes not to lengthen the intervals to >6 months in view of the relatively high incidence of concomitant PDAC. This is an obvious flaw of the Fukuoka consensus and large-scale prospective studies are awaited to solve a contradiction between those two statements. A French group reported a low incidence of malignant transformation and adequacy of lengthening of the follow-up intervals, but they still recommended biannual imaging studies (62). Tamura et al. (58) claimed that even a 6-month interval might not be sufficient to diagnose a concomitant PDAC in a patient with IPMN.
The length of surveillance for IPMN is another concern for every clinician. Although the Fukuoka consensus states that there are no good long-term data to indicate whether surveillance can be safely spaced to every 2 years or even discontinued after long-term stability, the guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts issued by the American Gastroenterology Association (AGA) in 2015 recommends stopping the surveillance of pancreatic cysts in 5 years of no significant changes, if high-risk features are completely negated and the patient does not have a strong family history of PDAC. They state that the small risk of malignant progression in stable cysts is likely outweighed by the costs of surveillance (63). However, there have not ever been any evidences reported on continuance or discontinuance of surveillance of IPMN.
It appears that the pancreas may be affected by “field carcinogenesis” in patients with IPMN. IPMN is quite often multiple as reported as up to 83% (64). Multiple IPMNs, ≥10 in number, were reported to be associated with higher prevalence of HGD or invasive carcinoma including concomitant PDAC (65). Moreover, even multifocal PDACs may be present in patients with IPMN (66). The “field carcinogenesis” may also give rise to PDAC even after resection of invasive or noninvasive IPMN or concomitant PDAC, requiring life-long close surveillance (52,55,64). In this regard, the AGA guideline will need to be revised in the near future. As the “field carcinogenesis” of the pancreas may have some relationship with multiple IPMNs and concomitant pancreatic intraepithelial neoplasia (PanIN) or PDAC in patients with familial PDAC (67), a family history of PDAC should be carefully taken as the AGA guideline states as well.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183-97. [DOI] [PubMed] [Google Scholar]
- 2.Ohhashi K, Tajiri H, Gondo M, et al. A case of cystadenocarcinoma of the pancreas forming bilio-pancreatic fistula. Prog Dig Endosc 1980;17:261-4. [Google Scholar]
- 3.Ohhashi K, Murakami F, Maruyama M. Four cases of mucous secreting pancreatic cancer. Prog Digest Endosc 1982;203:348-51. [Google Scholar]
- 4.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17-32. [DOI] [PubMed] [Google Scholar]
- 5.Nagai E, Ueki T, Chijiiwa K, et al. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called "mucinous ductal ectasia". Am J Surg Pathol 1995; 19:576-89. [DOI] [PubMed] [Google Scholar]
- 6.Nakata K, Ohuchida K, Aishima S, et al. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas 2011;40:581-7. [DOI] [PubMed] [Google Scholar]
- 7.Akita H, Takeda Y, Hoshino H, et al. Mural nodule in branch duct-type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am J Surg 2011;202:214-9. [DOI] [PubMed] [Google Scholar]
- 8.Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol 2013;11:913-21. [DOI] [PubMed] [Google Scholar]
- 9.Kim KW, Park SH, Pyo J, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg 2014;259:72-81. [DOI] [PubMed] [Google Scholar]
- 10.Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg 2009;249:628-34. [DOI] [PubMed] [Google Scholar]
- 11.Hirono S, Tani M, Kawai M, et al. Treatment strategy for intraductal papillary mucinous neoplasm of the pancreas based on malignant predictive factors. Arch Surg 2009;144:345-9. [DOI] [PubMed] [Google Scholar]
- 12.Hirono S, Tani M, Kawai M, et al. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2012;255:517-22. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Yamaue H, Maguchi H, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 2013;42:883-8. [DOI] [PubMed] [Google Scholar]
- 14.Uehara H, Ishikawa O, Katayama K, et al. Size of mural nodule as an indicator of surgery for branch duct intraductal papillary mucinous neoplasm of the pancreas during follow-up. J Gastroenterol 2011;46:657-63. [DOI] [PubMed] [Google Scholar]
- 15.Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas 2010;39:232-6. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M. Controversies in the management of pancreatic IPMN. Nat Rev Gastroenterol Hepatol 2011;8:56-60. [DOI] [PubMed] [Google Scholar]
- 17.Wong J, Weber J, Centeno BA, et al. High-grade dysplasia and adenocarcinoma are frequent in side-branch intraductal papillary mucinous neoplasm measuring less than 3 cm on endoscopic ultrasound. J Gastrointest Surg 2013;17:78-84; discussion p.84-5. [DOI] [PubMed]
- 18.Shindo K, Ueda J, Aishima S, et al. Small-sized, flat-type invasive branch duct intraductal papillary mucinous neoplasm: a case report. Case Rep Gastroenterol 2013;7:449-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritz S, Klauss M, Bergmann F, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg 2012;256:313-20. [DOI] [PubMed] [Google Scholar]
- 20.Koshita S, Fujita N, Noda Y, et al. Invasive carcinoma derived from "flat type" branch duct intraductal papillary mucinous neoplasms of the pancreas: impact of classification according to the height of mural nodule on endoscopic ultrasonography. J Hepatobiliary Pancreat Sci 2015;22:301-9. [DOI] [PubMed] [Google Scholar]
- 21.Goh BK, Tan DM, Thng CH, et al. Are the Sendai and Fukuoka Consensus Guidelines for cystic mucinous neoplasms of the pancreas useful in the initial triage of all suspected pancreatic cystic neoplasms? A single-institution experience with 317 surgically-treated patients. Ann Surg Oncol 2014;21:1919-26. [DOI] [PubMed] [Google Scholar]
- 22.Kang MJ, Jang JY, Kim SJ, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol 2011;9:87-93. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu Y, Kanemitsu Y, Sano T, et al. A nomogram for predicting the probability of carcinoma in patients with intraductal papillary-mucinous neoplasm. World J Surg 2010;34:2932-8. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsuka T, Kono H, Nagayoshi Y, et al. An increase in the number of predictive factors augments the likelihood of malignancy in branch duct intraductal papillary mucinous neoplasm of the pancreas. Surgery 2012;151:76-83. [DOI] [PubMed] [Google Scholar]
- 25.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan Pancreas Society. Pancreas 2011;40:67-71. [DOI] [PubMed] [Google Scholar]
- 26.Hirooka Y, Goto H, Itoh A, et al. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol 2003;18:1323-4. [DOI] [PubMed] [Google Scholar]
- 27.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology 2013;13:48-57. [DOI] [PubMed] [Google Scholar]
- 28.Mikata R, Ishihara T, Tada M, et al. , Clinical usefulness of repeated pancreatic juice cytology via endoscopic naso-pancreatic drainage tube in patients with pancreatic cancer. J Gastroenterol 2013;48:866-73. [DOI] [PubMed] [Google Scholar]
- 29.Sai JK, Suyama M, Kubokawa Y, et al. Pancreatic-duct-lavage cytology in candidates for surgical resection of branch-duct intraductal papillary mucinous neoplasm of the pancreas: should the International Consensus Guidelines be revised? Gastrointest Endosc 2009;69:434-40. [DOI] [PubMed] [Google Scholar]
- 30.Hara T, Ikebe D, Odaka A, et al. Preoperative histological subtype classification of intraductal papillary mucinous neoplasms (IPMN) by pancreatic juice cytology with MUC stain. Ann Surg 2013;257:1103-11. [DOI] [PubMed] [Google Scholar]
- 31.Genevay M, Mino-Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg 2011;254:977-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono J, Yaeger KA, Genevay M, et al. Cytological analysis of small branch-duct intraductal papillary mucinous neoplasms provides a more accurate risk assessment of malignancy than symptoms. Cytojournal 2011;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitman MB, Michaels PJ, Deshpande V, et al. Cytological and cyst fluid analysis of small (< or =3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology 2008;8:277-84. [DOI] [PubMed] [Google Scholar]
- 34.Wilentz RE, Chung CH, Sturm PD, et al. K-ras mutations in the duodenal fluid of patients with pancreatic carcinoma. Cancer 1998;82:96-103. [DOI] [PubMed] [Google Scholar]
- 35.Mori Y, Ohtsuka T, Kono H, et al. A minimally invasive and simple screening test for detection of pancreatic ductal adenocarcinoma using biomarkers in duodenal juice. Pancreas 2013;42:187-92. [DOI] [PubMed] [Google Scholar]
- 36.Iguchi H, Sugano K, Fukayama N, et al. Analysis of Ki-ras codon 12 mutations in the duodenal juice of patients with pancreatic cancer. Gastroenterology 1996;110:221-6. [DOI] [PubMed] [Google Scholar]
- 37.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013;11:719-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka M, Yokohata K, Konomi H, et al. Segmental balloon cytology for preoperative localization of in situ pancreatic cancer. Gastrointest Endosc 1997;46:447-9. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi K, Nakamura K, Yokohata K, et al. Pancreatic cyst as a sentinel of in situ carcinoma of the pancreas. Report of two cases. Int J Pancreatol 1997;22:227-31. [DOI] [PubMed] [Google Scholar]
- 40.Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: A prospective study in 197 patients. Clin Gastroenterol Hepatol 2006;4:1265-70. [DOI] [PubMed] [Google Scholar]
- 41.Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 2008;57:1561-5. [DOI] [PubMed] [Google Scholar]
- 42.Ingkakul T, Sadakari Y, Ienaga J, et al. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg 2010;251:70-5. [DOI] [PubMed] [Google Scholar]
- 43.Tanno S, Nakano Y, Sugiyama Y, et al. Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology 2010;10:173-8. [DOI] [PubMed] [Google Scholar]
- 44.Ikeuchi N, Itoi T, Sofuni A, et al. Prognosis of cancer with branch duct type IPMN of the pancreas. World J Gastroenterol 2010;16:1890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: A multicenter study in Japan. Pancreas 2011;40:364-70. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka M. Thirty years of experience with intraductal papillary mucinous neoplasm of the pancreas: From discovery to international consensus. Digestion 2014;90:265-72. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka M. Current roles of endoscopy in the management of intraductal papillary mucinous neoplasm of the pancreas. Dig Endosc 2015;27:450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi K, Ohuchida J, Ohtsuka T, et al. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology 2002;2:484-90. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 2011;40:571-80. [DOI] [PubMed] [Google Scholar]
- 50.Sey MS, Teagarden S, Settles D, et al. Prospective Cross-Sectional Study of the Prevalence of Incidental Pancreatic Cysts During Routine Outpatient Endoscopic Ultrasound. Pancreas 2015;44:1130-3. [DOI] [PubMed] [Google Scholar]
- 51.Tanno S, Nakano Y, Koizumi K, et al. Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 2010;39:36-40. [DOI] [PubMed] [Google Scholar]
- 52.Ohtsuka T, Kono H, Tanabe R, et al. Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am J Surg 2012;204:44-8. [DOI] [PubMed] [Google Scholar]
- 53.Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct IPMNs of the pancreas. Pancreatology 2012;12:198-202. [DOI] [PubMed] [Google Scholar]
- 54.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg 2013;258:466-75. [DOI] [PubMed] [Google Scholar]
- 55.He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg 2013;216:657-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohtsuka T, Ideno N, Aso T, et al. Role of endoscopic retrograde pancreatography for early detection of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci 2013;20:356-61. [DOI] [PubMed] [Google Scholar]
- 57.Lafemina J, Katabi N, Klimstra D, et al. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol 2013;20:440-7. [DOI] [PubMed] [Google Scholar]
- 58.Tamura K, Ohtsuka T, Ideno N, et al. Unresectable pancreatic ductal adenocarcinoma in the remnant pancreas diagnosed during every-6-month surveillance after resection of branch duct intraductal papillary mucinous neoplasm: a case report. JOP 2013;14:450-3. [DOI] [PubMed] [Google Scholar]
- 59.Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol 2010;45:952-9. [DOI] [PubMed] [Google Scholar]
- 60.Sakamoto H, Kitano M, Komaki T, et al. Small invasive ductal carcinoma of the pancreas distinct from branch duct intraductal papillary mucinous neoplasm. World J Gastroenterol 2009;15:5489-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014;46:22-9. [DOI] [PubMed] [Google Scholar]
- 62.Arlix A, Bournet B, Otal P, et al. Long-term clinical and imaging follow-up of nonoperated branch duct form of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2012;41:295-301. [DOI] [PubMed] [Google Scholar]
- 63.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:819-22. [DOI] [PubMed] [Google Scholar]
- 64.Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2012;255:326-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raman SP, Kawamoto S, Blackford A, et al. Histopathologic findings of multifocal pancreatic intraductal papillary mucinous neoplasms on CT. AJR Am J Roentgenol 2013;200:563-9. [DOI] [PubMed] [Google Scholar]
- 66.Mori Y, Ohtsuka T, Tsutsumi K, et al. Multifocal pancreatic ductal adenocarcinomas concomitant with intraductal papillary mucinous neoplasms of the pancreas detected by intraoperative pancreatic juice cytology. A case report. JOP 2010;11:389-92. [PubMed] [Google Scholar]
- 67.Bartsch DK, Dietzel K, Bargello M, et al. Multiple small "imaging" branch-duct type intraductal papillary mucinous neoplasms (IPMNs) in familial pancreatic cancer: indicator for concomitant high grade pancreatic intraepithelial neoplasia? Fam Cancer 2013;12:89-96. [DOI] [PubMed] [Google Scholar]