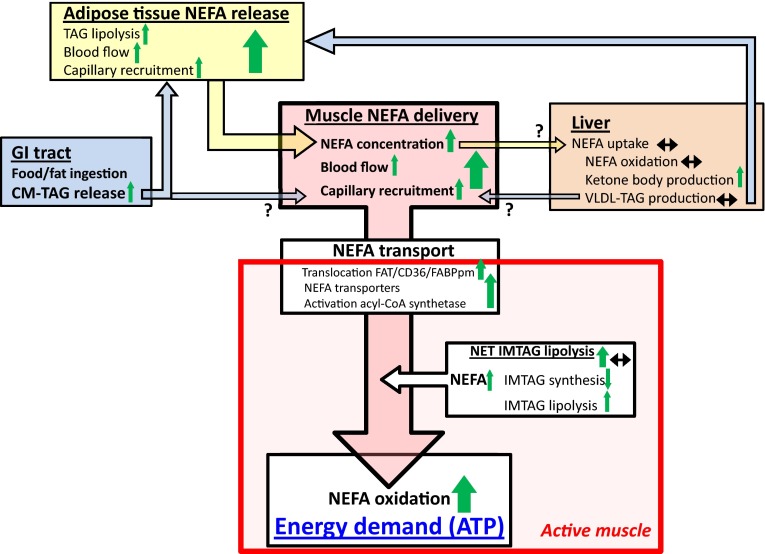
Fig. 1.
Schematic representation of the control of non-esterified fatty acid (NEFA) fluxes and oxidation during exercise described in terms of the integration of physiological systems. Central in the scheme is the close linear relationship between the active muscle NEFA delivery and uptake/oxidation observed during continuous moderate-intensity exercise. The increase in NEFA delivery is caused by an increase in plasma flow that includes capillary recruitment, whereby a larger portion of the total available NEFA transport machinery becomes accessible. Plasma flow and capillary recruitment do not change much with continuous exercise at the same workload; hence, the increase in muscle NEFA delivery and uptake/oxidation with exercise duration is mainly mediated by the increase in NEFA concentration. The increase in the plasma NEFA concentration is facilitated by an increased release of NEFA from adipose tissue via increased adipose tissue triacylglycerol (TAG) lipolysis, adipose tissue blood flow, and capillary recruitment with epinephrine and possible atrial natriuretic peptide as the key regulators during exercise. Liver NEFA uptake at rest and during exercise is substantial but does not seem to change much with exercise, thus it does not have much effect on the plasma NEFA concentration. Mandatory, but likely not limiting for NEFA oxidation in healthy individuals, is the facilitated NEFA uptake by transporters and binding proteins of which fatty acid translocase (FAT/CD36) and fatty acid bounding protein (FABPpm) translocate from the intracellular depot(s) to the plasma membrane with muscle contraction. NEFA from intramuscular TAG (IMTAG) is used during moderate-intensity exercise. The net degradation of IMTAG is caused by a decrease in IMTAG synthesis and maintained or increased lipolysis. Moreover, NEFA originating from either very-low-density lipoproteins (VLDL-TAG), or chylomicrons (CM-TAG) primarily in the fed state, may contribute to the active skeletal muscle NEFA oxidation. Dietary fat reaches the liver, adipose tissue, and skeletal muscle in the form of CM-TAG that undergoes lipolysis by lipoprotein lipase (LPL) and the resulting NEFAs are taken up and either oxidized or esterified by the active muscle (see also Fig. 2). The contribution of VLDL-TAG- and CM-TAG-derived NEFA to the resting and active muscle energy requirements seems limited. ATP adenosine triphosphate, CoA coenzyme A, GI gastrointestinal