Fig. 1. Serum binding activity to the pre-F and post-F conformations of the RSV F glycoprotein.
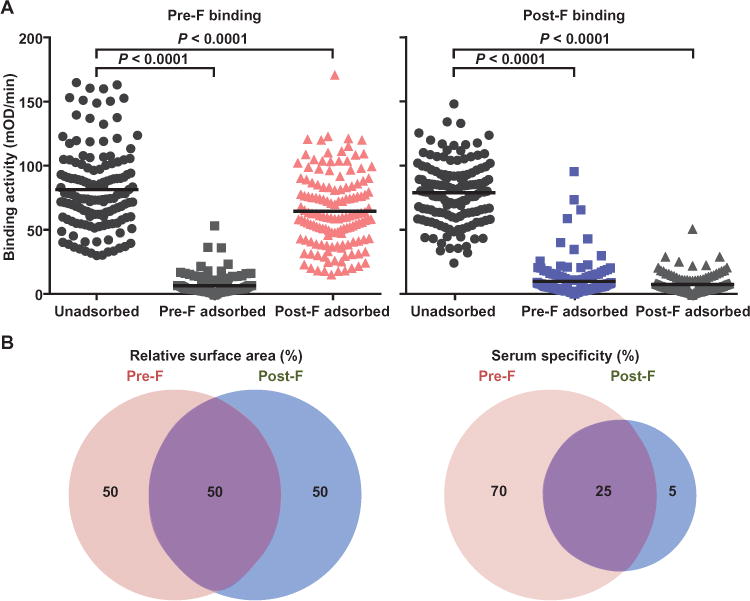
(A) Human sera were analyzed for binding to pre-F and post-F conformations by kinetic ELISA (black circles, left and right panels, respectively). Binding of unadsorbed sera to pre-F and post-F binding was similar (75.8 and 75.4 mOD/min, respectively). Sera were also analyzed for binding to either F conformation after adsorption with pre-F or post-F subunit proteins. As expected, pre-F adsorption removed antibodies that could bind to pre-F, and post-F adsorption removed antibodies that could bind post-F. Post-F adsorption did not remove a substantial amount of pre-F binding antibodies (pink triangles, left panel), but pre-F adsorption removed nearly all post-F binding antibodies (light blue squares, right panel). (B) Pre-F and post-F conformations share about 50% of their surface, and about 50% of the surface is unique to each. On the basis of the binding data, about 70% of antibodies in human sera bind pre-F–specific surfaces, about 25% bind the shared surfaces, and about 5% bind post-F–specific surfaces, which is disproportionate to the available surface area.
