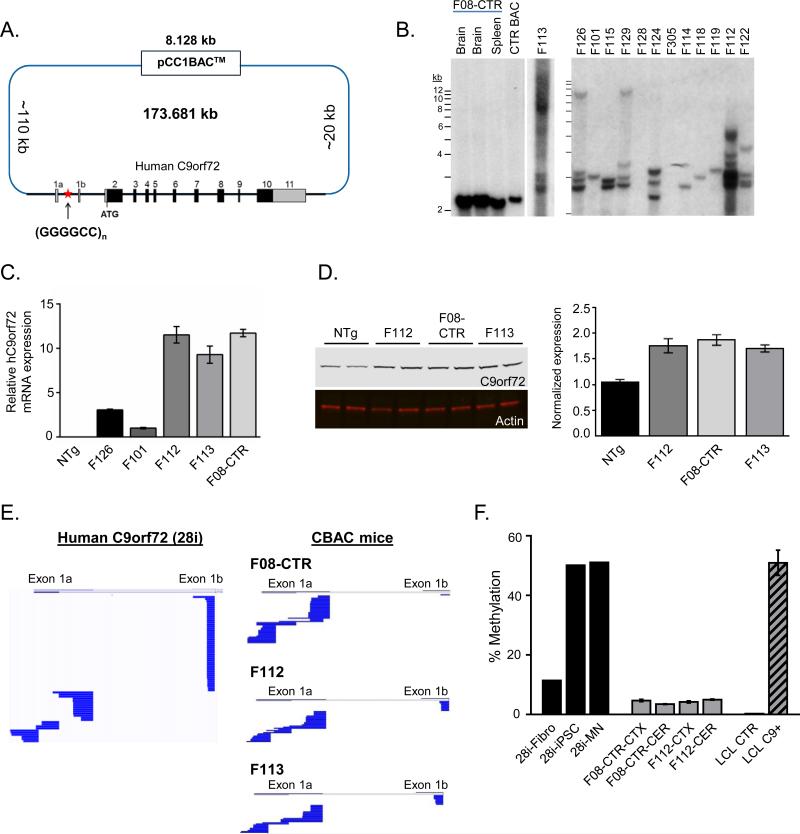
Figure 1. Generation of C9orf72 BAC transgenic mice.
(A) Schematic diagram of C9-BAC construct. The pCC1 BAC backbone (8.128 kb) was used to isolate a region contained the entire human C9orf72 gene (~36 kb) from a patient with C9-ALS, including ~110 kb upstream and ~20 kb downstream. >30 different C9-BAC founder lines were generated with either the contracted control BAC (15 repeats) or expansions of various sizes.
(B) Southern blot analysis of the repeat length in C9-BAC founder lines. A contracted subclone was used to generate the control (F08-CTR) line with 15 GGGGCC repeats. Lines F08-CTR, F112 and F113 showed comparable expression, and lines F112 and F113 had a variety of repeat sizes ranging from ~100-1000.
(C) Relative expression of human C9orf72 mRNA in cortex from C9-BAC transgenic lines measured by qPCR.
(D) Western blot analysis of C9orf72 protein (48kD) expressed in C9-BAC mouse cortex (left). Anti-C9orf72 (top), and anti-Actin (bottom). Western blot quantitation (right) normalized to nontransgenic (NTg) control.
(E) Sequence alignments of exon 1a and exon 1b utilization from 5’RACE analysis of C9orf72 transcripts. iPSC-derived motor neurons are shown on the left (from BAC donor patient) to demonstrate exon 1a and 1b utilization in human cells.
(F) C9orf72 promoter methylation assay as assessed by MSRE-qPCR. Fibroblasts (Fibro), induced pluripotent stem cells (iPSC) and iPSC-derived motor neurons (MN) are shown for BAC donor patient (~800 repeats), which revealed variable methylation from ~10% in fibroblasts to ~50% in iPSC and MNs. Methylation levels were low (~5%) in C9-BAC transgenic lines. Lymphoblastoid cell lines (LCL) from a non-expanded patient (CTR) and C9orf72 expansion carrier (C9+) are shown for comparison. *All data are shown as mean ± SEM. See also Figure S1.