Abstract
Background:
Cushing's disease is a condition rarely encountered during pregnancy. It is known that hypercortisolism is associated with increased maternal and fetal morbidity and mortality. When hypercortisolism from Cushing's disease does occur in pregnancy, the impact of achieving biochemical remission on fetal outcomes is unknown. We sought to clarify the impact of successful surgical treatment by presenting such a case report.
Case Description:
A 38-year-old pregnant woman with recurrent Cushing's disease after 8 years of remission. The patient had endoscopic transsphenoidal of her pituitary adenoma in her 18th week of pregnancy. The patient had postoperative biochemical remission and normal fetal outcome with no maternal complications.
Conclusion:
Transsphenoidal surgery for Cushing's disease can be performed safely during the second trimester of pregnancy.
Keywords: Cushing's syndrome, endonasal, endoscopic, fetal complications, minimally invasive skull base surgery, operative timing, pituitary
INTRODUCTION
The first published case of Cushing's syndrome in pregnancy was reported by Hunt and McConahey in 1953.[23] Since then, there have been around 200 cases reported in the literature. Cushing syndrome presenting in pregnancy is rare as hypercortisolism suppresses the secretion of gonadotropins and ovarian estrogen and progesterone, resulting in amenorrhea or oligomenorrhea in 75% of cases[19,28,49,55] Cushing's disease occurs in 60–70% of all patients with Cushing's syndrome, but occurs only in approximately 33% of the reported Cushing's syndrome cases in pregnancy.[6,21,29,40]
The diagnosis of Cushing's disease in pregnancy presents a challenge due to up regulation of the hypothalamic-pituitary axis (HPA) and associated hypercortisolism of pregnancy.[27] In this article, we present a case report of a patient who presented with recurrent Cushing's disease, became pregnant in the setting of hypercortisolism, and was subsequently successfully treated during pregnancy via an endoscopic endonasal transsphenoidal approach. To our knowledge, this is only the twelfth reported surgically treated case of Cushing's disease in pregnancy. The physiology of the HPA in pregnancy, diagnostic laboratory, and imaging, as well as neurosurgical, and anesthetic treatment considerations are also reviewed.
CASE DESCRIPTION
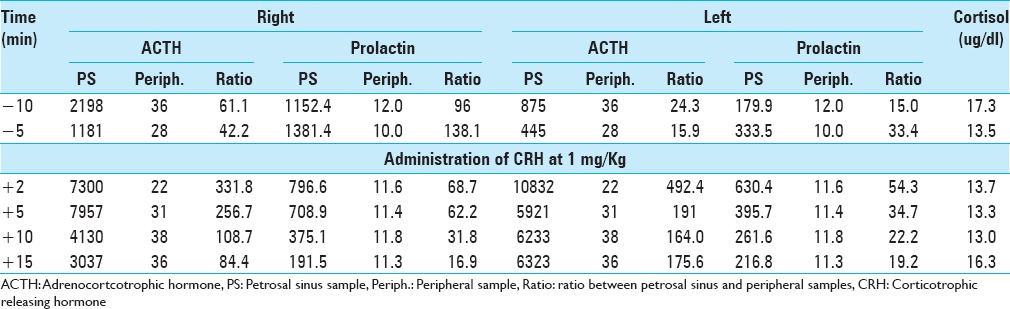
A 38-year-old pregnant woman presented with recurrent Cushing's disease. She had initially presented 8 years prior with weight gain, truncal obesity, and hypertension. Adrenocorticotrophic hormone (ACTH)-dependent hypercortisolism was confirmed. Urinary free cortisol (UFC) was 105.1 ug/24 h (reference range <45 ug/24 h), midnight serum cortisol was 249 ng/dl (reference range <100 ng/dl), and ACTH was 60 pg/ml (reference range 5–27 pg/ml). The patient had a negative high-resolution brain magnetic resonance imaging (MRI) with contrast. Inferior petrosal sinus sampling (IPSS) results were consistent with a pituitary source of her ACTH-dependent Cushing's syndrome [Table 1]. After discussion with the patient, she was taken to the operating room for sellar exploration. Extensive sellar exploration as well as serial vertical sections through the gland in approximately 2 mm intervals was performed through the entire anterior gland in addition to exploration of the posterior lobe. Several suggestive lesions were identified and removed. On postoperative day (POD) number 1, the patient's cortisol level dropped to 1.4 ug/dl from preoperative levels of 22.3 ug/dl. The patient was started on hydrocortisone 20 mg in the morning and 10 mg in the evening. The patient was discharged on POD number 3. Six months later in follow-up ACTH was 7 pg/ml (5–27 pg/ml). One-year after the first surgery the patient got pregnant, which resulted in a normal full-term neonate.
Table 1.
Inferior petrosal sinus sampling done at initial diagnosis before the first surgery
The patient remained in remission and re-presented after 8 years with symptoms of fatigue, diaphoresis, 50 pound (22.7 kg) weight gain over 3–4 months, easy bruising, hair loss, hypertension, and headaches. Biochemical workup was as follows: 24-h UFC 172.1 μg (reference range <45 ug/24 h), ACTH 56.3 pg/ml (reference range 6–48 pg/ml); 8 a.m. serum cortisol 26.9 μg/dl. Two consecutive midnight salivary cortisol samples were 245 and 262 ng/dl (reference range <100 ng/dl). Dedicated MRI of the pituitary with gadolinium contrast revealed a 4 mm hypointense sellar lesion that was suspicious for recurrent pituitary microadenoma [Figure 1].
Figure 1.
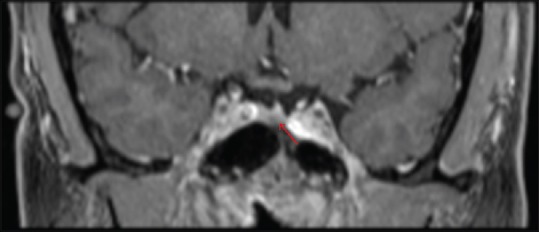
Magnetic resonance imaging brain with gadolinium contrast demonstrating a left 4 mm hypointense pituitary lesion (red arrow) that was suspicious for recurrent pituitary microadenoma
The patient was scheduled for elective surgery, but it was canceled as she was found to be pregnant on the day of the surgery, which corresponded to her 12th week of pregnancy (G4P3). After being re-evaluated by endocrinology and high-risk obstetrics, surgical resection was recommended during pregnancy in order to minimize potential life-threating maternal and fetal complications as a result of hypercortisolism. In addition, it was recommended that surgery be performed during the second trimester of pregnancy. After a thorough discussion of the risks and benefits to both her and the baby, she elected to proceed.
The patient underwent an image-guided endoscopic endonasal transsphenoidal approach during the 18th week of pregnancy. The patient was positioned in semilateral supine position [Figure 2] to avoid undue pressure of her gravid uterus on her iliac veins. After adequate exposure of the sphenoid sinus had been obtained, a wide sellar exposure was performed extending to both cavernous sinuses. Given the 9 mm intracarotid distance, extra time was spent confirming the carotid location with neuro-navigation and the Doppler probe. Dura was opened with a sickle knife. Scar tissue was found on the dura and intradurally. Notably, the pituitary gland had been sectioned during the previous operation, which made identification of abnormal tissue challenging. Biopsies were taken from far left lateral, left lateral, central, right lateral, and posterior parts of pituitary gland and sent to pathology. Frozen section from the left lateral aspect was suspicious for pituitary adenoma. No cerebrospinal fluid was encountered and the dural defect was covered by inlay dural substitute and onlay mucosal graft harvested from the middle turbinate. Postoperatively, the patient demonstrated normalization of her blood pressure and no longer required antihypertensives. Nadir cortisol and ACTH levels postoperatively on day 2 came back 1.1 ug/dl (reference range 3.4–26.9 ug/dl) and 6 pg/ml (reference range 6–48 pg/ml), respectively, and the patient was started on replacement hydrocortisone. She developed diabetes insipidus postoperatively, which has been controlled to date by oral desmopressin acetate 0.1 mg in the morning and 0.2 mg at bedtime. The patient delivered a full-term healthy neonate at her 39th week of gestation. The baby's weight was 8 lbs (3.6 kg). At 3 months follow-up, the patient is still on hydrocortisione replacement therapy and desmopressin 0.1 mg/day. ACTH stimulation test was 3.0 and 4.0 ug/dl at 30 and 60 min after CORTROSYN administration (a peak of at least 18 ug/dl is considered normal).
Figure 2.
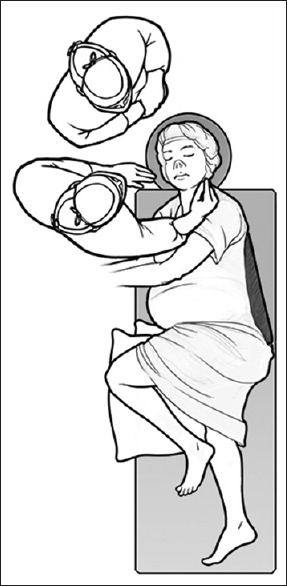
An illustration showing positioning for endoscopic transsphenoidal surgery in patients with gravid uterus
DISCUSSION
Physiology of the hypothalamic-pituitary-adrenal axis in pregnancy
The HPA axis function is upregulated during pregnancy. The placenta produces corticotropin-releasing hormone (CRH), which is structurally identical to hypothalamic CRH.[27] As a result, plasma CRH concentrations are nearly 1000 times higher in pregnancy compared with the nonpregnant state and normalize within 24 h of delivery.[17,28,45,46,47] CRH appears to be an important mediator of the “gestational clock” and early activation of placental CRH is associated with premature labor.[29,30,56] Cortisol first increases around the 11th week, peaks between the first and second trimester, and plateaus in the third trimester.[15,29,36,49] In pregnancy, serum cortisol and urine free cortisol are 2–3 times greater compared to nonpregnant controls.[27,49,50,57] ACTH also rises throughout pregnancy and normalizes within 2 h of delivery, whereas plasma cortisol takes longer to normalize.[7,27,38] In addition to increased CRH and ACTH, another contributing factor to hypercortisolism of pregnancy is decreased negative feedback on the pituitary gland by circulating cortisol. During the second and third trimester, 1 mg dexamethasone results in plasma cortisol suppression of 40% compared to 87% in nonpregnant controls.[37,48] The fetus is protected from increased maternal cortisol levels by placental 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2), which converts cortisol to inactive 11-keto metabolites.[27,48] In late gestation, there is reversal of 11β-HSD2, which may help in fetal lung maturation.[29,34] In physiologic hypercortisolism of pregnancy, there is preservation of the normal circadian rhythm in cortisol levels, although it may be slightly blunted compared to normal.[27]
Endocrinological diagnostic considerations
Clinical diagnosis of Cushing's syndrome in pregnancy is difficult due to an overlap in clinical features such as weight gain, fatigue, emotional change, abdominal striae, hypertension, and hyperglycemia.[25] In pregnant women with Cushing's syndrome, serum cortisol is between 2 and 22 times (median 8 times) the upper limit of normal.[25,27] Some authors have suggested that a UFC >3 times the upper limit of normal is a reliable indicator for the diagnosis of Cushing's syndrome in pregnancy.[25,26,44] Because circadian changes in cortisol levels are preserved in pregnancy, an elevated midnight salivary cortisol can also be helpful to diagnose Cushing's syndrome during pregnancy.[7,12,29,35] Due to the blunted negative feedback in pregnancy, overnight dexamethasone suppression tests have a high rate of false positive results.[29,55] IPSS can be used to differentiate a central versus ectopic source of excess ACTH in patients with ACTH-dependent Cushing's syndrome. It is rarely used in pregnancy due to the risks of ionizing radiation. However, if necessary, a direct jugular approach can be utilized in addition to abdominal lead barriers.[27,39]
Imaging of the pituitary gland in pregnancy
Detection of a pituitary adenoma on MRI can help confirm the diagnosis of Cushing's disease. However, imaging the pituitary gland during pregnancy requires awareness of special considerations. MRI is generally considered safe during pregnancy, although some authors have recommended avoiding MRI during the first trimester due to potential unknown adverse effects during organogenesis.[27] Gadolinium is categorized by the Food and Drug Administration (FDA) as category C and is generally avoided during pregnancy. It is important to note, however, that the sensitivity of MRI for detecting Cushing's disease in the nonpregnant population significantly decreases from 52% with gadolinium to 38% without gadolinium.[51] In addition, the pituitary gland undergoes a natural hyperplasia during pregnancy. The pituitary gland volume increases beginning at 12 weeks and doubles in size by the end of the third trimester.[18] In pregnancy, the gland does not exceed a vertical height of 10 mm, however, it may reach 12 mm in the immediate postpartum period. After the immediate postpartum period, the gland rapidly returns to normal size, regardless of breast-feeding status.[16] In addition, a normal anterior pituitary lobe in pregnancy tends to have a homogenous signal on T1-weighted images.[18]
Neurosurgical and anesthetic considerations
Given the high rate of maternal and fetal complications from untreated Cushing's syndrome, treatment is usually justified during pregnancy. The goal of treatment is to reduce UFC to the upper part of normal observed in pregnancy.[4,25] Prior studies have demonstrated superior maternal and fetal outcomes in addition to a higher rate of live births when medical treatment is implemented in the second trimester compared to no intervention during pregnancy.[25,29] The second trimester is considered the optimal time for surgical intervention because anesthesia given in the first trimester carries an increased risk of spontaneous abortion,[10,43] whereas anesthesia in the third trimester carries an elevated risk of premature labor.[10,52] Transsphenoidal surgery has been successfully performed in the second trimester in a small number of published case reports.[8,13,25,31,40,41,42,54]
Anesthetic considerations include ensuring adequate uterine blood flow, avoidance of anesthetic agents with possible teratogenic effects, and avoidance of hypoxia and acidosis.[1] The normal physiologic changes happening during pregnancy as well as pathophysiologic changes during Cushing's syndrome are important to consider when planning the general anesthetic needed. Difficulty in managing the airway as well as a high chance of aspiration could be a result of both pregnancy and Cushing's syndrome.[31] Uterine weight may compress the inferior vena cava and decrease preload and lead to arterial hypotension and placental insufficiency. A wedge underneath the right hip can displace the uterus and mitigate this effect.[14] Care must be taken to avoid undue pressure on the uterus by the surgeon or by any equipment during the case. Adequate hydration and special attention to fluid and electrolyte balance is important. Induced arterial hypotension which is often used in pituitary surgery to avoid excessive bleeding from the nasal mucosa should be avoided. Alternatively, local vasoconstrictive agents, warm saline nasal irrigation, and various thrombin containing hemostatic agents can be used instead. Although continuous fetal monitoring in conjunction with high-risk obstetric surveillance is recommended and definitely an option, it is probably best when it is utilized in situations where there is a viable fetus, thus giving immediate information about fetal well-being and if there is a need for immediate intervention including delivery.[5,14,32] The mean arterial blood pressure should first be increased if any intraoperative fetal compromise is detected.[32,53] For a fetus that has not reached a viable age, preinduction, and postsurgical monitoring will provide sufficient information. Regardless of timing, close and continuous communication between the obstetrician, perinatologist, anesthesiologist, and the surgeon is essential to ensuring the best outcome.[1]
Medical therapy
Although surgery is the first line treatment option for pregnant patients with Cushing's disease, medical therapy should be considered if surgery is not feasible. Despite being classified as a Class C drug by the FDA, meytrapone is the most commonly used medical therapy and is generally well-tolerated.[9,11] However, it does carry risk of fetal hypoadrenalism and increased risk of maternal hypertension and preeclampsia.[5,20,22] Ketoconazole, also FDA category C, has been used in few case reports, but is not generally used due to animal studies which demonstrated that it crosses the placenta and is both teratogenic and abortifacient.[2,3,24,33] In pregnancies in which medical therapy has been instituted, transient neonatal adrenal insufficiency should be anticipated and treated, as it is life-threatening and easily treatable.[19]
CONCLUSION
We present a case of successful surgical treatment of recurrent Cushing's disease during pregnancy. Biochemical remission was achieved in this case during pregnancy and the outcome was a healthy full-term neonate with no maternal complications during pregnancy or labor. Transsphenoidal surgery for Cushing's disease can be performed safely during the second trimester of pregnancy but requires extra vigilance and close communication between sub-specialty team members.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Mahmoud Abbassy, Email: abbassyma@gmail.com.
Varun R. Kshettry, Email: varunkshettry@gmail.com.
Amir H. Hamrahian, Email: hamraha@ccf.org.
Philip C. Johnston, Email: pcjohnston@doctors.org.uk.
Georgianna A. Dobri, Email: dobrig@ccf.org.
Rafi Avitsian, Email: avitsir@ccf.org.
Troy D. Woodard, Email: woodart@ccf.org.
Pablo F. Recinos, Email: recinop@ccf.org.
REFERENCES
- 1.Abd-Elsayed AA, Diaz-Gomez J, Barnett GH, Kurz A, Inton-Santos M, Barsoum S, et al. A case series discussing the anaesthetic management of pregnant patients with brain tumours. F1000Res. 2013;2:92. doi: 10.12688/f1000research.2-92.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berwaerts J, Verhelst J, Mahler C, Abs R. Cushing's syndrome in pregnancy treated by ketoconazole: Case report and review of the literature. Gynecol Endocrinol. 1999;13:175–82. doi: 10.3109/09513599909167552. [DOI] [PubMed] [Google Scholar]
- 3.Boronat M, Marrero D, López-Plasencia Y, Barber M, Schamann Y, Nóvoa FJ. Successful outcome of pregnancy in a patient with Cushing's disease under treatment with ketoconazole during the first trimester of gestation. Gynecol Endocrinol. 2011;27:675–7. doi: 10.3109/09513590.2010.521268. [DOI] [PubMed] [Google Scholar]
- 4.Boscaro M, Arnaldi G. Approach to the patient with possible Cushing's syndrome. J Clin Endocrinol Metab. 2009;94:3121–31. doi: 10.1210/jc.2009-0612. [DOI] [PubMed] [Google Scholar]
- 5.Bronstein MD, Salgado LR, de Castro Musolino NR. Medical management of pituitary adenomas: The special case of management of the pregnant woman. Pituitary. 2002;5:99–107. doi: 10.1023/a:1022364514971. [DOI] [PubMed] [Google Scholar]
- 6.Buescher MA, McClamrock HD, Adashi EY. Cushing syndrome in pregnancy. Obstet Gynecol. 1992;79:130–7. [PubMed] [Google Scholar]
- 7.Carr BR, Parker CR, Jr, Madden JD, MacDonald PC, Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139:416–22. doi: 10.1016/0002-9378(81)90318-5. [DOI] [PubMed] [Google Scholar]
- 8.Casson IF, Davis JC, Jeffreys RV, Silas JH, Williams J, Belchetz PE. Successful management of Cushing's disease during pregnancy by transsphenoidal adenectomy. Clin Endocrinol (Oxf) 1987;27:423–8. doi: 10.1111/j.1365-2265.1987.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 9.Close CF, Mann MC, Watts JF, Taylor KG. ACTH-independent Cushing's syndrome in pregnancy with spontaneous resolution after delivery: Control of the hypercortisolism with metyrapone. Clin Endocrinol (Oxf) 1993;39:375–9. doi: 10.1111/j.1365-2265.1993.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Kerem R, Railton C, Oren D, Lishner M, Koren G. Pregnancy outcome following non-obstetric surgical intervention. Am J Surg. 2005;190:467–73. doi: 10.1016/j.amjsurg.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Connell JM, Cordiner J, Davies DL, Fraser R, Frier BM, McPherson SG. Pregnancy complicated by Cushing's syndrome: Potential hazard of metyrapone therapy. Case report. Br J Obstet Gynaecol. 1985;92:1192–5. doi: 10.1111/j.1471-0528.1985.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 12.Cousins L, Rigg L, Hollingsworth D, Meis P, Halberg F, Brink G, et al. Qualitative and quantitative assessment of the circadian rhythm of cortisol in pregnancy. Am J Obstet Gynecol. 1983;145:411–6. doi: 10.1016/0002-9378(83)90309-5. [DOI] [PubMed] [Google Scholar]
- 13.Coyne TJ, Atkinson RL, Prins JB. Adrenocorticotropic hormone-secreting pituitary tumor associated with pregnancy: Case report. Neurosurgery. 1992;31:953–5. doi: 10.1227/00006123-199211000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Day J, Batjer HH. Operative positioning and fetal monitoring considerations. In: Loftus M, editor. Neurosurgical Aspects of Pregnancy. Illinois: American Association of Neurological Surgeons; 1995. pp. 183–90. [Google Scholar]
- 15.Demey-Ponsart E, Foidart JM, Sulon J, Sodoyez JC. Serum CBG, free and total cortisol and circadian patterns of adrenal function in normal pregnancy. J Steroid Biochem. 1982;16:165–9. doi: 10.1016/0022-4731(82)90163-7. [DOI] [PubMed] [Google Scholar]
- 16.Elster AD, Sanders TG, Vines FS, Chen MY. Size and shape of the pituitary gland during pregnancy and post partum: Measurement with MR imaging. Radiology. 1991;181:531–5. doi: 10.1148/radiology.181.2.1924800. [DOI] [PubMed] [Google Scholar]
- 17.Goland RS, Wardlaw SL, Blum M, Tropper PJ, Stark RI. Biologically active corticotropin-releasing hormone in maternal and fetal plasma during pregnancy. Am J Obstet Gynecol. 1988;159:884–90. doi: 10.1016/s0002-9378(88)80162-5. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez JG, Elizondo G, Saldivar D, Nanez H, Todd LE, Villarreal JZ. Pituitary gland growth during normal pregnancy: An in vivo study using magnetic resonance imaging. Am J Med. 1988;85:217–20. doi: 10.1016/s0002-9343(88)80346-2. [DOI] [PubMed] [Google Scholar]
- 19.Gopal RA, Acharya SV, Bandgar TR, Menon PS, Shah NS. Cushing disease with pregnancy. Gynecol Endocrinol. 2012;28:533–5. doi: 10.3109/09513590.2011.632789. [DOI] [PubMed] [Google Scholar]
- 20.Gormley MJ, Hadden DR, Kennedy TL, Montgomery DA, Murnaghan GA, Sheridan B. Cushing's syndrome in pregnancy – Treatment with metyrapone. Clin Endocrinol (Oxf) 1982;16:283–93. doi: 10.1111/j.1365-2265.1982.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 21.Guilhaume B, Sanson ML, Billaud L, Bertagna X, Laudat MH, Luton JP. Cushing's syndrome and pregnancy: Aetiologies and prognosis in twenty-two patients. Eur J Med. 1992;1:83–9. [PubMed] [Google Scholar]
- 22.Hána V, Dokoupilová M, Marek J, Plavka R. Recurrent ACTH-independent Cushing's syndrome in multiple pregnancies and its treatment with metyrapone. Clin Endocrinol (Oxf) 2001;54:277–81. doi: 10.1046/j.1365-2265.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 23.Hunt AB, McConahey WM. Pregnancy associated with diseases of the adrenal glands. Am J Obstet Gynecol. 1953;66:970–87. doi: 10.1016/s0002-9378(16)38611-2. [DOI] [PubMed] [Google Scholar]
- 24.Leiba S, Weinstein R, Shindel B, Lapidot M, Stern E, Levavi H, et al. The protracted effect of o, p’-DDD in Cushing's disease and its impact on adrenal morphogenesis of young human embryo. Ann Endocrinol (Paris) 1989;50:49–53. [PubMed] [Google Scholar]
- 25.Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK. Cushing's syndrome during pregnancy: Personal experience and review of the literature. J Clin Endocrinol Metab. 2005;90:3077–83. doi: 10.1210/jc.2004-2361. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay JR, Nieman LK. Adrenal disorders in pregnancy. Endocrinol Metab Clin North Am. 2006;35:1–20, v. doi: 10.1016/j.ecl.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay JR, Nieman LK. The hypothalamic-pituitary-adrenal axis in pregnancy: Challenges in disease detection and treatment. Endocr Rev. 2005;26:775–99. doi: 10.1210/er.2004-0025. [DOI] [PubMed] [Google Scholar]
- 28.Magiakou MA, Mastorakos G, Webster E, Chrousos GP. The hypothalamic-pituitary-adrenal axis and the female reproductive system. Ann N Y Acad Sci. 1997;816:42–56. doi: 10.1111/j.1749-6632.1997.tb52128.x. [DOI] [PubMed] [Google Scholar]
- 29.McCarroll F, Lindsay JR. Cushing's syndrome in pregnancy. In: Swearingen B, Biller BM, editors. Cushing's Disease. New York: Springer; 2011. [Google Scholar]
- 30.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–3. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 31.Mellor A, Harvey RD, Pobereskin LH, Sneyd JR. Cushing's disease treated by trans-sphenoidal selective adenomectomy in mid-pregnancy. Br J Anaesth. 1998;80:850–2. doi: 10.1093/bja/80.6.850. [DOI] [PubMed] [Google Scholar]
- 32.Molitch ME. Pituitary tumors and pregnancy. Growth Horm IGF Res. 2003;13(Suppl A):S38–44. doi: 10.1016/s1096-6374(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 33.Moudgal VV, Sobel JD. Antifungal drugs in pregnancy: A review. Expert Opin Drug Saf. 2003;2:475–83. doi: 10.1517/14740338.2.5.475. [DOI] [PubMed] [Google Scholar]
- 34.Murphy BE. Conversion of cortisol to cortisone by the human uterus and its reversal in pregnancy. J Clin Endocrinol Metab. 1977;44:1214–7. doi: 10.1210/jcem-44-6-1214. [DOI] [PubMed] [Google Scholar]
- 35.Newell-Price J, Trainer P, Perry L, Wass J, Grossman A, Besser M. A single sleeping midnight cortisol has 100% sensitivity for the diagnosis of Cushing's syndrome. Clin Endocrinol (Oxf) 1995;43:545–50. doi: 10.1111/j.1365-2265.1995.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 36.Nolten WE, Rueckert PA. Elevated free cortisol index in pregnancy: Possible regulatory mechanisms. Am J Obstet Gynecol. 1981;139:492–8. doi: 10.1016/0002-9378(81)90331-8. [DOI] [PubMed] [Google Scholar]
- 37.Odagiri E, Ishiwatari N, Abe Y, Jibiki K, Adachi T, Demura R, et al. Hypercortisolism and the resistance to dexamethasone suppression during gestation. Endocrinol Jpn. 1988;35:685–90. doi: 10.1507/endocrj1954.35.685. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto E, Takagi T, Makino T, Sata H, Iwata I, Nishino E, et al. Immunoreactive corticotropin-releasing hormone, adrenocorticotropin and cortisol in human plasma during pregnancy and delivery and postpartum. Horm Metab Res. 1989;21:566–72. doi: 10.1055/s-2007-1009289. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 40.Pickard J, Jochen AL, Sadur CN, Hofeldt FD. Cushing's syndrome in pregnancy. Obstet Gynecol Surv. 1990;45:87–93. doi: 10.1097/00006254-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Pinette MG, Pan YQ, Oppenheim D, Pinette SG, Blackstone J. Bilateral inferior petrosal sinus corticotropin sampling with corticotropin-releasing hormone stimulation in a pregnant patient with Cushing's syndrome. Am J Obstet Gynecol. 1994;171:563–4. doi: 10.1016/0002-9378(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 42.Ross RJ, Chew SL, Perry L, Erskine K, Medbak S, Afshar F. Diagnosis and selective cure of Cushing's disease during pregnancy by transsphenoidal surgery. Eur J Endocrinol. 1995;132:722–6. doi: 10.1530/eje.0.1320722. [DOI] [PubMed] [Google Scholar]
- 43.Sam S, Molitch ME. Timing and special concerns regarding endocrine surgery during pregnancy. Endocrinol Metab Clin North Am. 2003;32:337–54. doi: 10.1016/s0889-8529(03)00012-4. [DOI] [PubMed] [Google Scholar]
- 44.Sammour RN, Saiegh L, Matter I, Gonen R, Shechner C, Cohen M, et al. Adrenalectomy for adrenocortical adenoma causing Cushing's syndrome in pregnancy: A case report and review of literature. Eur J Obstet Gynecol Reprod Biol. 2012;165:1–7. doi: 10.1016/j.ejogrb.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT. Immunoreactive corticotropin-releasing factor is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab. 1984;59:812–4. doi: 10.1210/jcem-59-4-812. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki A, Shinkawa O, Yoshinaga K. Immunoreactive corticotropin-releasing hormone in amniotic fluid. Am J Obstet Gynecol. 1990;162:194–8. doi: 10.1016/0002-9378(90)90848-2. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki A, Tempst P, Liotta AS, Margioris AN, Hood LE, Kent SB, et al. Isolation and characterization of a corticotropin-releasing hormone-like peptide from human placenta. J Clin Endocrinol Metab. 1988;67:768–73. doi: 10.1210/jcem-67-4-768. [DOI] [PubMed] [Google Scholar]
- 48.Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 2000;57:1412–7. doi: 10.1046/j.1523-1755.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 49.Sheeler LR. Cushing's syndrome and pregnancy. Endocrinol Metab Clin North Am. 1994;23:619–27. [PubMed] [Google Scholar]
- 50.Suda T, Iwashita M, Ushiyama T, Tozawa F, Sumitomo T, Nakagami Y, et al. Responses to corticotropin-releasing hormone and its bound and free forms in pregnant and nonpregnant women. J Clin Endocrinol Metab. 1989;69:38–42. doi: 10.1210/jcem-69-1-38. [DOI] [PubMed] [Google Scholar]
- 51.Tabarin A, Laurent F, Catargi B, Olivier-Puel F, Lescene R, Berge J, et al. Comparative evaluation of conventional and dynamic magnetic resonance imaging of the pituitary gland for the diagnosis of Cushing's disease. Clin Endocrinol (Oxf) 1998;49:293–300. doi: 10.1046/j.1365-2265.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 52.Terhune KP, Jagasia S, Blevins LS, Jr, Phay JE. Diagnostic and therapeutic dilemmas of hypercortisolemia during pregnancy: A case report. Am Surg. 2009;75:232–4. [PubMed] [Google Scholar]
- 53.Tuncali B, Aksun M, Katircioglu K, Akkol I, Savaci S. Intraoperative fetal heart rate monitoring during emergency neurosurgery in a parturient. J Anesth. 2006;20:40–3. doi: 10.1007/s00540-005-0359-4. [DOI] [PubMed] [Google Scholar]
- 54.Verdugo C, Alegría J, Grant C, Briano E, González MI, Meza H, et al. Cushing's disease treatment with transsphenoidal surgery during pregnancy. Rev Med Chil. 2004;132:75–80. doi: 10.4067/s0034-98872004000100012. [DOI] [PubMed] [Google Scholar]
- 55.Vilar L, Freitas Mda C, Lima LH, Lyra R, Kater CE. Cushing's syndrome in pregnancy: An overview. Arq Bras Endocrinol Metabol. 2007;51:1293–302. doi: 10.1590/s0004-27302007000800015. [DOI] [PubMed] [Google Scholar]
- 56.Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079–85. doi: 10.1016/s0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 57.Wallace C, Toth EL, Lewanczuk RZ, Siminoski K. Pregnancy-induced Cushing's syndrome in multiple pregnancies. J Clin Endocrinol Metab. 1996;81:15–21. doi: 10.1210/jcem.81.1.8550743. [DOI] [PubMed] [Google Scholar]


