Figure 5.
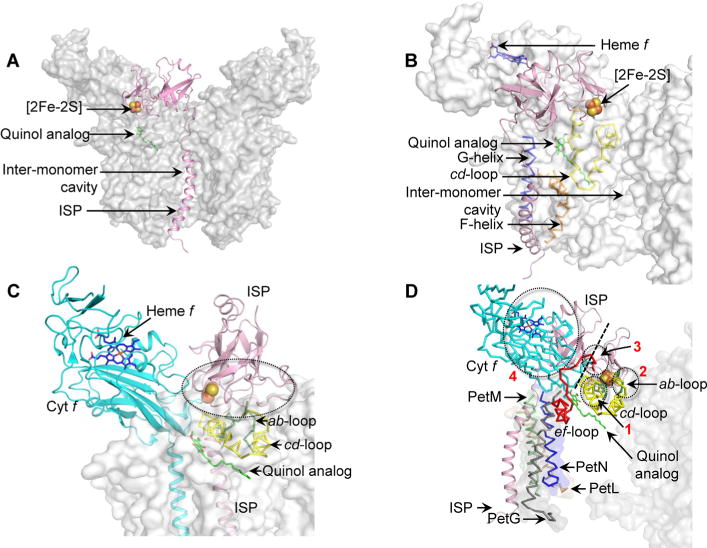
Models of steric hindrance in ISP interactions when ISP is inserted in a non-native position within the cyt b6f complex (PDB entry 4OGQ). (A) Location of the ISP TMH (pink) within the intermonomer cavity partially occludes the quinone/quinol exchange function of the cavity. (B) Insertion of the ISP TMH (pink) between the TMH F (orange ribbon) and G (blue ribbon) leads to extensive clashes between the ISP extrinsic domain and the cd loop (yellow ribbon) of cyt b6. (C) The ISP subunit within the same monomer of the cyt b6f complex orients the ISP extrinsic domain within unfavorable proximity of the cd loop (yellow), which leads to extensive clashes between the extrinsic domain and the cd loop. Regions of steric clashes are highlighted with a dotted oval. (D) Placement of the ISP TMH proximal to the PetG-L-M-N subunits located on the periphery of the cyt b6f complex. In such a location, the TMH-proximal N-terminal ISP subdomain undergoes extensive clashes with the cyt f extrinsic domain (cyan), while the distal, [2Fe-2S] binding C-terminal ISP subdomain clashes with the ab loop (green), the cd loop (yellow), and the ef loop (red). Regions of steric clashes are highlighted by three dotted ovals (1, clash with cd loop; 2, clash with ab and cd loops; 3, clash with ef loop; 4, clash with cyt f). A dotted black line separates the N-terminal, helix-proximal subdomain and the C-terminal cluster binding subdomain. For the sake of simplicity, the cyt b6f complex is shown as a gray surface. The Qp site is marked by the quinol analogue TDS molecule (green and red sticks, superposed from PDB entry 2E76). Loops ab (green, Phe56–Asn77), cd (yellow, Ser137–Gln177), and ef (red, Pro65–Asn93) are shown in the PyMol “ribbon” format, while the ISP and cyt f subunits are depicted in the PyMol “cartoon” format.
