Abstract
We present a rare finding of concurrent right testis non-seminomatous mixed germ cell tumor and muscle invasive urothelial carcinoma of the bladder in a 57-year-old homeless man. The socioeconomic factors and the disease presentation caused a treatment dilemma in terms of the appropriate type of neoadjuvant chemotherapy. The patient ultimately underwent upfront surgery with retroperitoneal lymph node dissection and radical cystoprostatectomy followed by adjuvant cisplatin-based chemotherapy.
Keywords: Urinary bladder neoplasm, Testis neoplasm
Introduction
The estimated incidence of bladder cancer in the year 2014 in US was much higher in comparison to the incidence of testis cancer (74,000 vs 8400 cases, respectively).1 To our knowledge, the synchronous occurrence of primary urothelial carcinoma of bladder (UCB) and testis cancer has not been previously reported. Separately, each of these malignancies has well established treatment algorithms, but the coexisting tumors in the current case presented an interesting dilemma regarding the most appropriate approach of therapy and the order in which it should occur.
Case report
A 57-year-old homeless man presented with complaints of intermittent painless gross hematuria and an enlarging right testicular mass. His medical history included epididymitis in the same testicle 7 years earlier, as well as tobacco (30 pack-year), cocaine and heroin abuse. He denied weight loss or back pain. On physical exam, he was found to have a hard, non-tender, right scrotal mass without overlying skin discoloration or erythema.
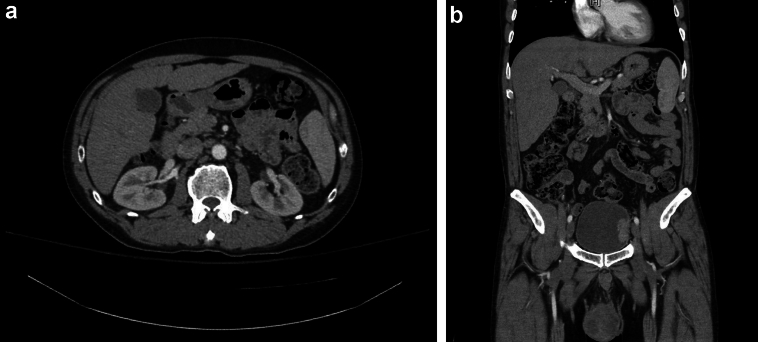
The scrotal ultrasound demonstrated a large heterogeneous mass with internal blood flow. A Computed Tomography (CT) of abdomen/pelvis revealed an enhancing mass measuring 5.1 cm at the left lateral bladder wall (Fig. 1) and multiple suspicious lymph nodes (5–10 mm) in the inter-aortocaval and periaortic regions, as well as a 6.6 cm right testis mass (Fig. 1). Initial laboratory findings were normal, except for serum tumor markers, which revealed both an elevated alpha-fetoprotein (AFP) (41.2 ng/mL) and lactate dehydrogenase (LDH) (312 units/L) with normal beta-human chorionic gonadotropin (β-HCG) (<1 mIU/mL).
Figure 1.
CT demonstrating: a) inter-aortocaval lymph node, b) left lateral bladder wall mass and right testicle mass.
Initially, the patient underwent a concurrent right radical orchiectomy and transurethral resection of the bladder tumor. The morphologic features along with the immunohistochemical profiles were consistent with a mixed germ cell tumor: mature and immature teratoma (80%), embryonal carcinoma (10%), and yolk sac tumor (10%). The tumor extended to the tunica albuginea and epididymis with lymphatic space involvement, pT2 stage (TNM). His AFP came down to normal levels after 4 weeks (2.4 ng/mL), but the LDH rose slightly to 320 units/L. In terms of bladder mass, pathology revealed high grade, muscle invasive urothelial carcinoma consistent with cT2 stage (TNM).
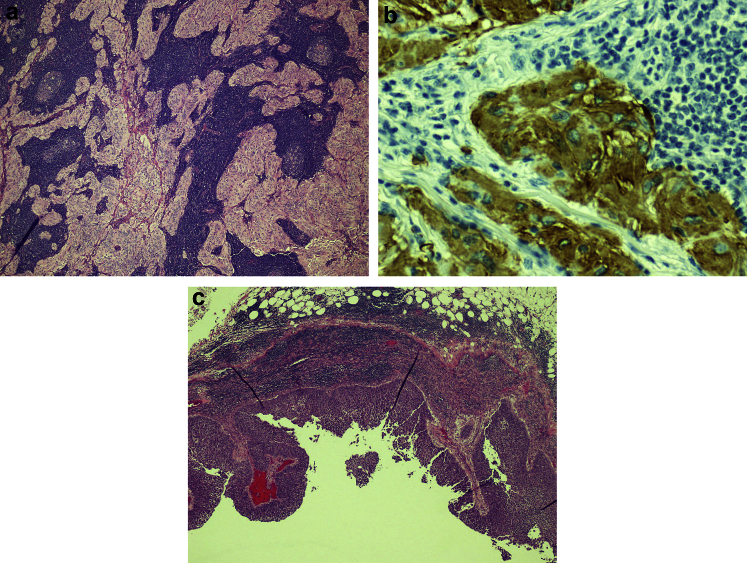
Treatment options were considered and discussed with the patient. Initial neoadjuvant chemotherapy with subsequent cystoprostatectomy may have been appropriate treatment for the muscle invasive UCB. Given the patient's history of non-compliance, as well as the concurrent NSGCT with aggressive features, prompt surgical treatment was planned with bilateral template retroperitoneal lymph node dissection (RPLND), radical cystoprostatectomy, and pelvic lymph node dissection (PLND). Final pathology after RPLND revealed a total of 44 lymph nodes, where one left para-aortic and one inter-aortocaval lymph node were positive for mature teratoma (Fig. 2). Final pathology after radical cystectomy showed invasive urothelial carcinoma into the outer half of the muscularis propria of the bladder, negative resection margins. A total of 21 pelvic lymph nodes were removed, of which one left external iliac lymph node was positive for metastatic urothelial carcinoma, with extranodal extension, stage pT2b, N1 (Fig. 2).
Figure 2.
Pathology slide of metastatic RPLN showing a) teratoma (hematoxylin and eosin stain), and b) Glial Fibrillary Acidic Protein (GFAP) stain consistent with glial origin of teratoma tissue; c) metastatic urothelial carcinoma in pelvic lymph node (hematoxylin and eosin stain).
Discussion
In terms of testis cancer, our patient was classified as good risk, CS IIA NSGCT (American Joint Committee on Cancer).2 However, CT finding of suspicious retroperitoneal nodes (5–10 mm) as well as presence of lymphovascular invasion and embryonal component in the primary testis tumor placed our patient at higher risk for occult metastasis.3, 4 It has been reported that the rate of metastasis when both entities are present varies from 30 to 90%, and in the absence of the two, the risk of metastasis is <20%.4 Embryonal carcinoma has been considered to be the most undifferentiated cell type of NSGCT, with totipotential ability to become other NSGCTs, including teratoma at primary or metastatic sites. The definition of embryonal predominance in higher risk patients varies in literature between 45 and 90%.4 In our patient, it was reported at 10% but its presence may still portend to its metastatic potential. A majority (80%) of our patient's primary testis tumor was noted to be teratoma, which is a chemo- and radio-resistant tumor only amenable to cure by surgical excision, which further influenced our treatment decision-making.
The established initial treatment options for Stage IIA NSGCT include either induction chemotherapy with Etoposide-Cisplatin (EP) ×4 or Bleomycin-Etoposide-Cisplatin (BEP) ×3, or primary RPLND. Any one of these approaches results in survival rates exceeding 95%, especially with the advent of cisplatin-based combination chemotherapy.4 Previously reported high 5-year progression-free probability of 86% in patients with pathologic stage N1 after RPLND alone, however, supported a surgical approach over primary chemotherapy.4 This approach allows a large portion of patients to avoid systemic chemotherapy and its well described long-term side effects. In addition, in considering the patient's mixed germ cell tumor with potential teratoma elements at metastatic sites, complete cure would require surgery to remove the teratomatous components. For our homeless patient, compliance with upfront chemotherapy and follow-up posed a significant problem. Furthermore, the coexistence of his bladder and testicular malignancies presented a challenge in the selection of an appropriate chemotherapy regimen.
Current level 1 evidence for treatment of MI-UCB demonstrates a survival advantage in patients receiving neoadjuvant chemotherapy with methotrexate, vinblastine, doxorubicin and cisplatin (MVAC), or gemcitabine and cisplatin (GC), followed by radical cystectomy.5 The role of adjuvant chemotherapy remains uncertain due to lack of prospective randomized trials, but some meta-analyses reports have shown a benefit in patient overall and disease-free survivals. After a discussion with the patient and a multidisciplinary team at our institution, initial surgical intervention including a combined bilateral template RPLND with radical cystoprostatectomy and PLND was performed. With the finding of teratoma in his nodes, RPLND allowed our patient to be potentially cured. Clearly, the benefit of initial surgical approach also provided more definitive staging and guidance for further chemotherapy. Metastatic UCB unfortunately does not share the same success with testis cancer. As a result, our final recommendation for our patient with coexisting metastatic urothelial and testis cancers favors early adjuvant MVAC chemotherapy and observation (conservative imaging and tumor markers) without adjuvant chemotherapy, respectively.
Conflicts of interest
None declared.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Edge S.B., Byrd D.R., Compton C.C., editors. AJCC cancer staging manual. 7th ed. Springer; New York, YN: 2010. [Google Scholar]
- 3.Leibovitch L., Foster R.S., Kopecky K.K., Donohue J.P. Improved accuracy of computerized tomography based clinical staging in low stage nonseminomatous germ cell cancer using size criteria of retroperitoneal lymph nodes. J Urol. 1995;154:1759–1763. [PubMed] [Google Scholar]
- 4.Choueiri T.K., Stephenson A.J., Gilligan T., Klein E.A. Management of clinical stage I nonseminomatous germ cell testicular cancer. Urol Clin North Am. 2007;34:137–148. doi: 10.1016/j.ucl.2007.02.001. abstract viii. [DOI] [PubMed] [Google Scholar]
- 5.Grossman H.B., Natale R.B., Tangen C.M. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]