Abstract
Stromal tumors of uncertain malignant potential (STUMP) are rare diagnoses in the evaluation of elevated PSA. The management of STUMP in the setting of an elevated PSA is challenging, as STUMP may have a benign clinical course. In this report, we describe a patient who was found to have a PSA >500 ng/ml and a large STUMP. We review the critical findings and review the relevant literature on diagnosis and management strategies for patients with STUMP.
Keywords: STUMP, Prostate, Elevated PSA
Introduction
The diagnosis of STUMP presents a diagnostic and management challenge for the practicing urologist. These tumors exhibit atypical stromal proliferation and can exhibit an unpredictable clinical course. Furthermore, the clinical and laboratory findings associated with STUMP may be nonspecific, leading to delayed diagnosis. Thus, the decision-making surrounding treatment of STUMP remains poorly understood.
Case presentation
The patient is a 63 year old healthy African American male without a family history of prostate cancer who was referred by his PCP with a history of benign prostate biopsies and a rising prostate specific antigen (PSA) level. Review of his medical records revealed a PSA of 58 ng/ml in 2004; 75 ng/ml in 2006; 106 ng/ml in 2009; and 90 ng/ml in 2011. Following his PSA in 2011, his local urologist performed a transrectal prostate biopsy, which revealed patchy fascicular spindle cell proliferation with CD34 positivity and negative PR, actin, desmin, and pankeratin on immunohistochemistry. Computed tomography and technetium bone scan failed to identify any evidence of metastatic disease. He continued to be followed conservatively with a PSA of 267 ng/ml in 2014. By the time he presented to our clinic, his PSA was 504 ng/ml. His International Prostate Symptom Score (IPSS) was 8 of 35 and he denied any bothersome lower urinary tract symptoms.
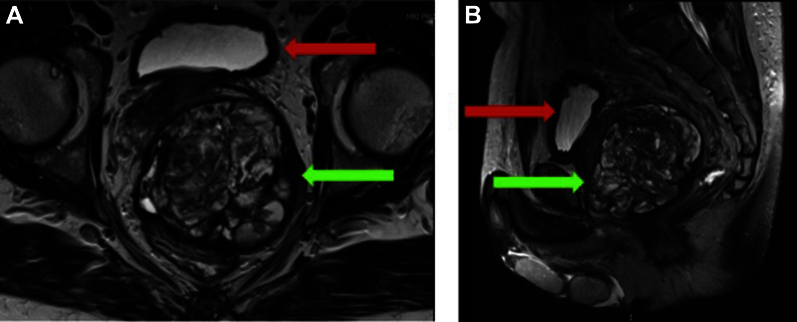
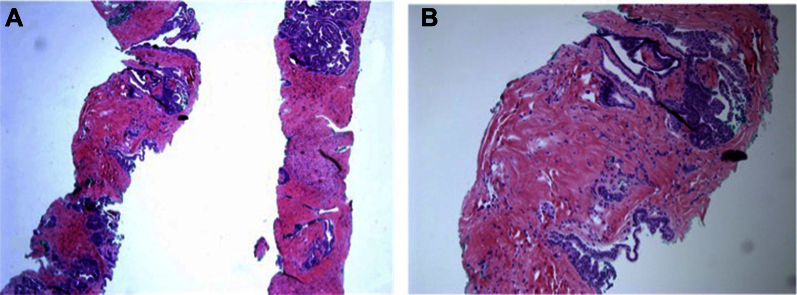
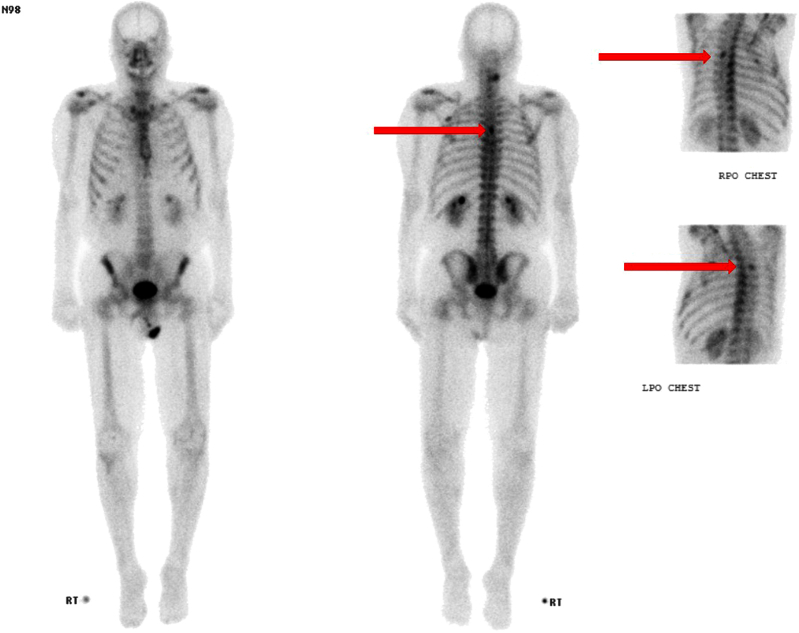
He underwent a prostate MRI, which confirmed a markedly enlarged prostate with an estimated volume of 290 cc. The differential diagnosis on MRI was sarcoma, STUMP, and adenocarcinoma (PIRADS 5/5) based on abnormal signal on T2-weighted imaging, ADC maps, and DCE throughout the gland (Fig. 1). There was suspected extraprostatic disease. His biopsy showed only subtle changes in the stroma without any prostatic carcinoma or stromal sarcoma identified (Fig. 2). A follow up bone scan showed degenerative changes with 2 nonspecific areas of osseous uptake in the fourth ribs and thoracic spine that were reported on a similar imaging study in 2011 (Fig. 3).
Figure 1.
Coronal (A) and sagittal (B) T2 magnetic resonance imaging demonstrating a large STUMP (green arrows) replacing the majority of the prostate and displacing the urinary bladder (red arrows).
Figure 2.
(A) Low power and (B) high power images of the transrectal prostate biopsy showing normal-appearing prostate glands and stroma without evidence of carcinoma or sarcoma.
Figure 3.
Whole body 99-Tm bone scan demonstrating non-specific activity in the T5 vertebra and bilateral posterior fourth ribs.
The findings were discussed with the patient including the potential for cystectomy and/or pelvic exenteration. He chose not to undergo surgery, recognizing the uncertain prognosis and possibility of bowel and urinary obstruction in the future.
Discussion
The diagnosis of prostate STUMP includes tumors with a spectrum of stromal proliferation and clinical presentations. As a result, diagnosis and management remains challenging. In an analysis of 50 patients with STUMP, stromal sarcoma, or mixed pathology, Herawi and Epstein identified urinary obstructive symptoms, abnormal digital rectal exam, hematuria, hematospermia, and rectal fullness as the patients' presenting symptoms and signs.1 Ages ranged from 27 to 83 years and elevated PSA was a motivating factor for biopsy in 11 of 50 cases. Reported tumor sizes ranged from 0.7 cm to 18 cm and weights ranged from 26 g to 1044 g.
Histologically, prostatic STUMP may have a variety of growth patterns and there are neither sensitive nor specific immunohistochemical markers. Indeed, Pan et al compared chromosomal imbalances between STUMP and stromal sarcomas. Of 14 specimens, common chromosomal losses included chromosome 13 (10 cases), chromosome 14 (9 cases), and chromosome 10 (7 cases).2 There were no observed differences in the chromosomal imbalances between STUMP and stromal sarcoma. Guadin et al proposed 4 subtypes – degenerative atypia, hypercellular, mixed type, and phyllodes type.3 In a review of 70 STUMP with a glandular component, Nagar and Epstein identified glandular crowding as the most common findings, seen in 50% of the tumors.4 The most common subtype seen was degenerative atypia, seen in 61% of the tumors. Seventeen percent of the tumors had glands that were identical to the uninvolved benign prostatic tissue. In the patients evaluated by Hewari and Epstein, prostate adenocarcinoma was found in 17 of 50 cases.1 Of the 15 patients who were treated with radical prostatectomy, 11 patients had adenocarcinoma as an incidental finding that was distinct from the stromal tumor.
Radiographically, there is limited literature on the appearance of STUMP. Nevertheless, these tumors may have varying findings, based on histological subtype and may be located in the peripheral and transition zones. Tumors that are multi-loculated, or have a necrotic or mucinous component may have higher T2 signal intensity.5 Solid lesions may be heterogeneous in appearance. Despite the atypical appearance, care must be taken to determine the location of the mass and its potential involvement of other pelvic structures.
Treatment of STUMP remains challenging. As a potentially benign entity, these tumors may be adherent to neighboring structures, making resection difficult and requiring extensive surgery for complete resection.1 STUMP may harbor stromal sarcoma, making incomplete resection a poor choice from an oncologic perspective. Previous efforts to manage these tumors with non-definitive resection have resulted in locally recurrent disease, reported in up to 46% of patients, or metastatic disease.1, 3 STUMP without stromal sarcoma may follow a benign course. However, in the cohort reported by Hewari and Epstein, 1 of 7 patients with mixed STUMP and sarcoma developed abdominal metastases and died of disease after almost 10 years following cystoprostatectomy. Another had para-aortic and bone metastases following cystoprostatectomy and was alive 23 years following treatment. The remaining had no evidence of disease following treatment.
Conclusion
STUMP is a rare pathological finding with a variable clinical presentation and unpredictable clinical course. To the authors' knowledge, this represents the first reported case of an STUMP with a PSA >500 ng/ml. Clinicians must be aware that STUMP may resemble or contain prostate stromal sarcoma. There are no guidelines for treatment, but intervention should be pursued only after thorough discussion on the risks and benefits of intervention.
Conflicts of interest
None to report.
References
- 1.Herawi M., Epstein J.I. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30(6):694–704. doi: 10.1097/00000478-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Pan C.C., Epstein J.I. Common chromosomal aberrations detected by array comparative geomic hybridization in specialized stromal tumors of the prostate. Mod Pathol. 2013 Nov;26(11):1536–1543. doi: 10.1038/modpathol.2013.99. [DOI] [PubMed] [Google Scholar]
- 3.Gaudin P.B., Rosai J., Epstein J.I. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol. 1998;22(2):148–162. doi: 10.1097/00000478-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Nagar M., Epstein J.I. Epithelial proliferations in prostatic stromal tumors of uncertain malignant potential (STUMP) Am J Surg Pathol. 2011 Jun;35(6):898–903. doi: 10.1097/PAS.0b013e318214f2f2. [DOI] [PubMed] [Google Scholar]
- 5.Muglia V.F., Saber G., Maggioni G., Jr., Monteiro A.J. MRI findings of prostate stromal tumors of uncertain malignant potential: a case report. Br J Radiol. 2011 Oct;84(1006):e194–e196. doi: 10.1259/bjr/67699443. [DOI] [PMC free article] [PubMed] [Google Scholar]