Abstract
At synapses, the presynaptic release machinery is precisely juxtaposed to the postsynaptic neurotransmitter receptors. We studied the molecular mechanisms underlying this exquisite alignment at the C. elegans inhibitory synapses. We found that the sole C. elegans neuroligin homologue, NLG-1, localizes specifically at GABAergic postsynapses and is required for clustering the GABAA receptor UNC-49. Two presynaptic factors, Punctin/MADD-4, an ADAMTS-like extracellular protein, and Neurexin/NRX-1, act partially redundantly to recruit NLG-1 to synapses. In the absence of both MADD-4 and NRX-1, NLG-1 and GABAA receptors fail to cluster, and GABAergic synaptic transmission is severely compromised. Biochemically, we detect an interaction between MADD-4 and NLG-1, as well as between MADD-4 and NRX-1. Interestingly, the presence of NRX-1 potentiates binding between Punctin/MADD-4 and NLG-1, suggestive of a tripartite receptor ligand complex. We propose that presynaptic terminals induce postsynaptic receptor clustering through the action of both secreted ECM proteins and trans-synaptic adhesion complexes.
Keywords: neuroligin, neurexin, MADD-4/punctin, GABAergic synapses, neuromuscular junctions, C. elegans
INTRODUCTION
Precise apposition between the presynaptic release machinery and postsynaptic receptors ensures that neurotransmitters trigger rapid and reliable synaptic response. Several mechanisms contribute to the precise alignment of synaptic membranes. At the vertebrate neuromuscular junction (NMJ), motor axon-secreted agrin plays a critical role in synapse maturation by clustering postsynaptic acetylcholine receptors (Sanes and Lichtman, 1999). Direct interaction between calcium channels and synaptic laminin helps to precisely localize active zones at the NMJ (Nishimune et al., 2004). In the central nervous system, many “synaptic organizers” have been described. These organizers include trans-synaptic adhesion molecules like Neurexin and Neuroligin, secreted synaptic adaptor proteins (Cbln), as well as glia-derived factors like thrombospondin (Siddiqui and Craig, 2011). The synaptogenic activity of these molecules was typically studied in excitatory glutamatergic synapses in vitro.
Among the synaptic adhesion molecules, the best studied Neurexin and Neuroligin pair plays important roles in the maturation and function of synapses(Südhof, 2008). Neuroligin and Neurexin bind to each other, and are sufficient to induce pre- and postsynaptic differentiation, respectively, in vitro (Ichtchenko et al., 1995;,Dean et al., 2003;,Graf et al., 2004). Despite this well-established synaptogenic activity, Neuroligin and Neurexin do not seem to be required for synapse development in vertebrates (Varoqueaux et al., 2006), but might in other organisms, as suggested by studies in Drosophila (Li et al., 2007). Two of the four rodent neuroligins, NLGN1 and NLGN2, show preferential association with excitatory and inhibitory postsynapses, respectively (Song et al., 1999;,Varoqueaux et al., 2004). Importantly, the genetic relationship between each of the neuroligins and neurexins is not established in vertebrates due to high level of redundancy in each gene family. It is therefore not known whether neuroligins’ postsynaptic localization requires the function of neurexins. Indeed, in Drosophila, while loss of neurexin (dNrx) or neuroligin (dNlg1) causes similar synaptic defects, dNrx binding is not absolutely required for dNlg1 function (Banovic et al., 2010). The C. elegans genome encodes a single neuroligin ortholog, NLG-1, and a single neurexin ortholog, NRX-1. Previous studies have shown that the C. elegans nlg-1 mutant display sensory processing defects, and an impairment in retrograde signaling at the cholinergic NMJs,.
We took advantage of the relative simplicity of C. elegans to investigate the role of neuroligin at postsynaptic sites. We found that NLG-1 specifically localizes to GABAergic postsynaptic NMJs. Consistent with this localization, nlg-1 null mutants display reduced GABAA receptor clustering and a reduction in spontaneous inhibitory currents (mIPSCs) frequency and amplitude. Both defects were rescued by restoring NLG-1 expression in body wall muscles. Our results also indicate that NLG-1 relies on extracellular interactions for GABAA receptor clustering, and that its binding partner NRX-1 is dispensable for such a function. However, we find that in the absence of NRX-1 and Punctin/MADD-4, NLG-1 and GABAAR clustering is severely compromised.
RESULTS
Neuroligin clusters GABAA receptors at postsynaptic sites
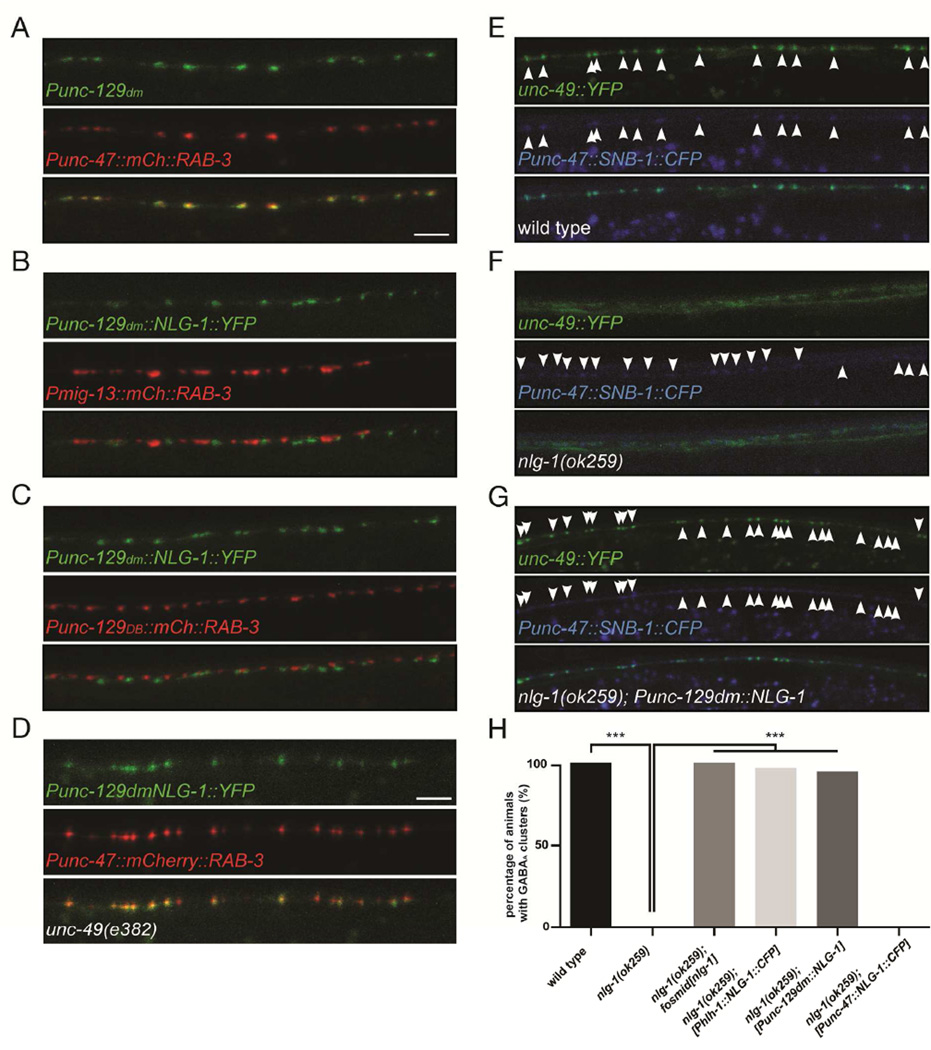
The C. elegans body wall muscles, which express NLG-1 (Hunter et al., 2010), receive direct synaptic inputs from both cholinergic and GABAergic motor neurons. To determine the subcellular localization of NLG-1 in muscles, we expressed a functional NLG-1::YFP (Hunter et al., 2010) using a muscle-specific promoter, and observed discrete puncta along the nerve cords (Fig. 1A), reminiscent of a postsynaptic distribution at NMJs. Colocalization analyses with GABAergic and cholinergic presynaptic markers revealed that NLG-1::YFP is precisely apposed to inhibitory presynaptic terminals, and is excluded from excitatory cholinergic synapses (Fig. 1A–C), similar to the specific NLGN2 localization at GABAergic postsynaptic terminals in mammals (Varoqueaux et al., 2004).
Figure 1. NLG-1 functions in muscles to cluster synaptic GABAA receptors.
(A—C) NLG-1::YFP puncta in muscle cells are apposed to GABAergic presynaptic terminals from the D-type motor neuron (A) in the dorsal nerve cord, and intercalate with excitatory cholinergic terminals from the A-type (B) or B-type (C) motor neurons. In all panels NLG-1 is expressed under the control of a fragment of the unc-129 promoter restricted to dorsal muscle cells, and the presynaptic marker mCherry::RAB-3 is expressed either from the D-type-specific unc-47 promoter (A), the DA9-specific promoter mig-13 (B), or the DB-specific promoter unc-129 (C). Arrowheads point to NLG-1::YFP puncta. (D) NLG-1::YFP clusters normally in unc-49(e382) animals lacking the GABAA receptor. (E) A YFP-tagged UNC-49 GABA receptor (top) forms clusters apposite to GABAergic presynaptic terminals labeled with a SNB-1::CFP marker (middle). (F) In nlg-1(ok259) mutants UNC-49::YFP is diffuse on the muscle cell plasma membrane (top). (G) Expression of NLG-1 specifically in muscle cells in nlg-1(ok259) animals fully rescues the UNC-49::YFP clustering defect. (H) Quantification of UNC-49::YFP clustering in wild type, nlg-1(ok259) mutants, and transgenic nlg-1(ok259) animals expressing NLG-1 under the control of various promoters. Expression of NLG-1 in muscles is sufficient to restore UNC-49::YFP clustering in nlg-1(ok259) mutants. Arrowheads point to SNB-1::CFP puncta. p<0.001, Fisher’s exact test. Scale bar: 5 µm.
The specific localization of muscle NLG-1 at inhibitory postsynaptic sites raises the possibility that it might play a role in the assembly and/or function of this synapse. The heteromultimeric GABAA receptor is composed of subunits encoded by the unc-49 locus in C. elegans (Richmond and Jorgensen, 1999;,Bamber et al., 1999). Therefore, we analyzed the distribution of the GABAA UNC-49 receptor in the absence of NLG-1. A fluorophore-tagged UNC-49B::YFP fusion protein forms clusters that appose the GABAergic presynaptic sites, labeled with SNB-1::CFP, similar to the antibody-labeled UNC-49 endogenous receptors (Fig. 1E and Gally and Bessereau, 2003). Interestingly, in nlg-1(ok259) animals, we observed diffuse YFP fluorescence outlining the muscle cell membranes (Fig. 1F and 1H), and failed to detect any obvious UNC-49B::YFP puncta above that diffuse fluorescence component, suggesting that UNC-49B::YFP properly reaches the plasma membrane but fails to cluster in the absence of NLG-1. SNB-1::CFP puncta were still visible on the presynaptic side (Fig. 1F). We asked whether GABAA receptors were reciprocally required for NLG-1 localization by examining NLG-1::YFP expression in the unc-49(e382) mutant background. The distribution of NLG-1::YFP clusters was not affected by the lack of UNC-49 (Fig. 1D), suggesting that NLG-1 lies upstream of the GABAA receptor in the postsynaptic assembly hierarchy, similar to observations made in vertebrate neurons (Patrizi et al., 2008).
Consistent with its postsynaptic localization, expression of NLG-1 or NLG-1::CFP under the control of two different muscle-specific promoters fully rescued the UNC-49B::YFP clustering defects, as did expression of NLG-1 under its endogenous promoter from a genomic fosmid (Fig. 1G and 1H). In contrast, expression of NLG-1 in the presynaptic inhibitory neurons did not restore UNC-49B::YFP clustering (Fig. 1H). These results suggest that NLG-1 functions cell autonomously in the body wall muscles to form or maintain GABAA receptor clusters at the inhibitory postsynapse. They are also consistent with the report that a subset of GABAergic postsynaptic specializations fail to form in the NLGN2−/− mutant mouse (Poulopoulos et al., 2009).
neuroligin mutants show defects in GABAergic synaptic transmission
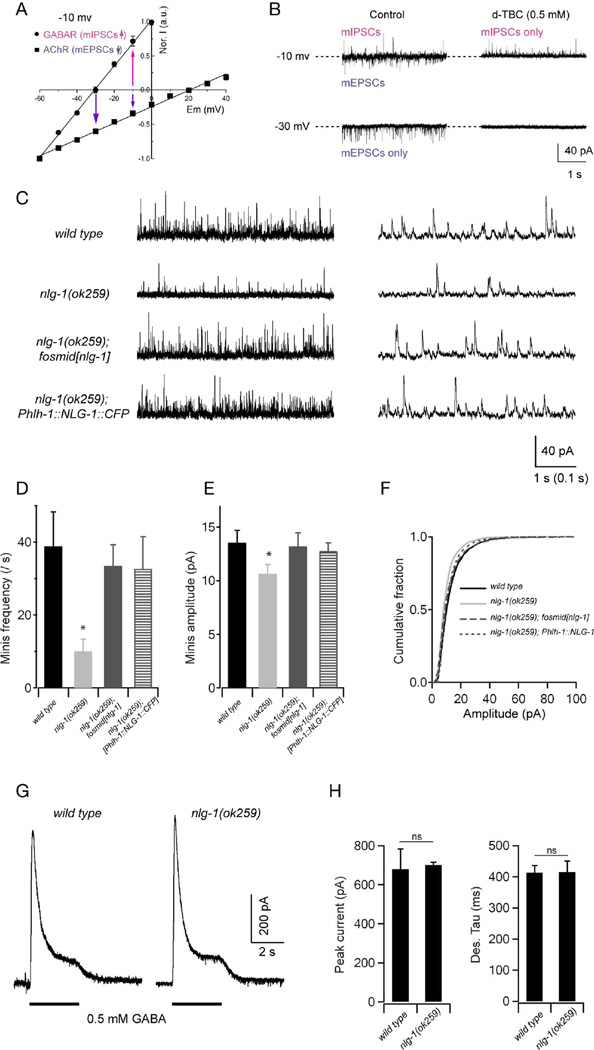
To investigate the functional consequence of the defective postsynaptic GABAA receptor clustering in nlg-1(ok259) mutants, we examined postsynaptic currents in body wall muscles through whole-cell patch clamp recording. Under our recording conditions, the reversal potentials of cholinergic and GABAergic receptors were +20 mV and −30 mV, respectively (Gao and Zhen, 2011; Fig. 2A). When the body wall muscles were held at −30 mV, only inward, spontaneous excitatory mEPSCs were detected (Fig. 2B). The identity of these mEPSCs was confirmed by their sensitivity to the application of the ionotropic acetylcholine receptor blocker D-tubocurarine (d-TBC, 0.5 mM) (Fig. 2B). When the muscle membrane potential was held at −10 mV, both inward (mEPSCs) and outward (mIPSCs) currents were recorded. The outward currents were insensitive to d-TBC, whereas the inward currents were blocked by d-TBC, confirming that the outward currents specifically represented GABAergic mIPSCs (Fig. 2B).
Figure 2. NLG-1 is required for normal GABAergic synaptic transmission.
Spontaneous inhibitory currents show reduced amplitude and frequency in nlg-1 mutants. (A,B) A protocol for recording GABAergic mIPSCs at the dissected muscle preparation. (A) In the recording solutions, the reversal potentials are around −30 mV and +20 mV for muscular GABAR and AChR, respectively. Adapted from Gao and Zhen, 2011. (B) Representative traces of spontaneous mIPSCs and mEPSCs in wild-type animals. When held at −10 mV, both mIPSCs and mEPSCs were recorded, as outward and inward currents, respectively. Application of 0.5 mM D-tubocurarine (d-TBC) allowed for the isolation of mIPSC events as it completely blocked mEPSCs. At −30 mV only mEPSCs were recorded (left bottom panel), since all events were blocked by 0.5 mM d-TBC (right bottom panel). (C) Representative traces with different time scales of spontaneous GABAergic mIPSCs in wild type, nlg-1(ok259), and transgenic nlg-1(ok259) mutants expressing NLG-1 under its endogenous promoter (nlg-1 fosmid), or a muscle-specific promoter (Phlh-1::NLG-1). Muscles were held at −10 mV. (D—F) Both the frequency and amplitude of mIPSCs were significantly decreased in nlg-1 mutants, and were restored to wild-type levels by expressing NLG-1 under its endogenous promoter, or a muscle-specific promoter. (G) Representative traces of GABA (0.5 mM) evoked currents at muscle cells. (H) The peak amplitude and desensitization kinetics of GABA-evoked currents in nlg-1 mutants were comparable to that in wild-type animals. * p<0.05, ns: not significant, by Mann-Whitney U test.
Using this protocol, we found that both the frequency and amplitude of mIPSCs were markedly reduced in nlg-1(ok259) mutants, as compared to wild-type animals (Fig. 2C–F). These defects could result from a decreased release probability at presynaptic terminals, or from a reduction of GABAA receptors on the postsynaptic membrane. Because these defects were fully rescued to a wild-type level by expressing NLG-1::CFP specifically in muscles (Fig. 2C–F), and because the presynaptic GABAergic terminals appear normal when examined with synaptic vesicle markers, we favor the latter explanation. It can be noted that overexpression of NLG-1 did not lead to an increase in mIPSCs frequency, suggesting that NLG-1 might not be a rate limiting factor for the inhibitory miniature currents.
To verify whether NLG-1 is specifically required for the clustering of the GABAA receptors, or for its insertion on the plasma membrane, we tested the evoked response in muscles upon exogenous GABA (0.5 mM) application. We found no significant difference in the amplitude or kinetics of the evoked GABAergic currents in nlg-1(ok259) mutants and wild-type animals, suggesting that functional GABA receptors are present on muscle cell membranes in nlg-1 mutant (Fig. 2G and 2H).
Together with the visual phenotype observed with the UNC-49::YFP marker, these results strongly argue that NLG-1 is crucial to cluster surface GABA receptors at postsynaptic sites. Interestingly, a similar reduction in spontaneous inhibitory postsynaptic currents has been described in NL2 knock-out mice (Poulopoulos et al., 2009), and more recently in mice models of ASD-associated NL3 mutations (Rothwell et al., 2014).
Consistent with a previous report (Hunter et al., 2010), we did not observe obvious locomotory behavioral phenotype in these animals (data not shown). This finding raises questions on the necessity of GABA signaling for C. elegans locomotion. A complete abolishment of GABAergic synaptic transmission (e.g. the unc-25/GAD or unc-49/GABAa mutants) leads to the “shrinker” phenotype, where animals exhibit hypercontraction, but retain the ability for sinusoidal movements (McIntire et al., 1993). Hence, it is possible that some GABAA receptor molecules form clusters that are beyond our detection threshold with the GABAAR::YFP marker, and sufficient to drive normal synaptic function during locomotion.
NLG-1 localizes to inhibitory synapses independently of postsynaptic scaffold proteins and of its known ligand NRX-1
Neuroligins are known to interact with a variety of extracellular and intracellular partners, including neurexins, thrombospondin and postsynaptic scaffolding molecules like PSD-95, S-SCAM and gephyrin (Poulopoulos et al., 2009;,Irie et al., 1997;,Iida et al., 2004;,Xu et al., 2010). It is unclear whether any of these interactions, alone or in concert, is necessary to form or maintain neuroligin clusters at postsynaptic sites in vivo. To investigate mechanisms for NLG-1 clustering at the postsynaptic NMJs, we first took a structure-function approach. We expressed NLG-1 deletion constructs that lack particular domains and analyzed their localization pattern. NLG-1, like its vertebrate homologs, is a single, type I transmembrane protein with an extracellular cholinesterase-like domain and a small intracellular tail ending with a PDZ-binding motif. To evaluate the importance of interactions through the PDZ-binding domain for NLG-1 localization, we first expressed a NLG-1::YFP construct that lacks the three most C-terminus amino acids. The corresponding NLG-1ΔPDZBD::YFP protein showed normal, punctate localization apposite to the GABAergic presynaptic terminals (Suppl. Fig. 1A). More surprisingly, we found that truncating the entire cytosolic tail (NLG-1ΔintraC::YFP) did not alter the localization of NLG-1::YFP (Suppl. Fig. 1B). Moreover, the ability of NLG-1ΔintraC::YFP to cluster normally was not due to dimerization with the endogenous NLG-1, since its localization was indistinguishable between wild-type and nlg-1(ok259) animals (Suppl. Fig. 1B and 1C). These results suggest that the recruitment of NLG-1 to inhibitory postsynaptic sites is entirely dependent on extracellular interactions. To further test this possibility, we expressed a truncated NLG-1 construct that retained the signal peptide directly followed by the extracellular YFP tag, the putative O-glycosylation region as well the transmembrane domain and cytosolic tail. The NLG-1ΔextraC::YFP did not form any detectable clusters, but was instead diffusely distributed on the plasma membrane (Suppl. Fig. 1D). Together, these results strongly argue that NLG-1 clusters can form independently of postsynaptic scaffolding protein interaction, but relies on extracellular binding partner(s). We also tested the ability of these truncated forms of NLG-1 to cluster GABAA receptors. Not surprisingly, NLG-1ΔextraC, which showed a diffuse distribution on the membrane, was unable to rescue the GABAAR clustering defect of nlg-1 mutants (Suppl. Fig 1F). Interestingly, just removing the 3-residue PDZ-binding motif at the C-terminus of NLG-1 drastically compromised its ability to cluster GABAA receptors (Suppl. Fig 1F). However NLG-1ΔintraC, which also clustered normally at inhibitory postsynapses, completely failed to rescue the GABAAR clustering defects (Suppl. Fig 1F), suggesting that other domains within the cytosolic tail of NLG-1 are necessary for GABA receptors recruitment. Together, this structure-function analysis shows that NLG-1 is recruited to GABAergic synapses via its extracellular domain and clusters GABAA receptors through its PDZ-binding motif and cytoplasmic tail.
The most likely trans-synaptic binding candidate for NLG-1 is the sole C. elegans neurexin ortholog, NRX-1, which has been reported to be expressed in most if not all C. elegans neurons (Haklai-Topper et al., 2011). The C. elegans nrx-1 locus encodes a long and a short neurexin isoforms. The long isoform encodes proteins that are similar to α-neurexins in vertebrates with a single transmembrane domain and a large extracellular domain encompassing multiple laminin G and EGF-like domains. The shorter isoform is generated from an alternative internal promoter in the same loci. To test if NRX-1 clusters NLG-1, we introduced 3 analogous missense mutations in NLG-1 that are predicted to abolish its putative binding sites for β-neurexin (Araç et al., 2007), and expressed the corresponding protein, NLG-1(QED), in muscle cells of nlg-1(ok259) mutant animals. The localization of NLG-1(QED) apposite to GABAergic presynaptic terminals was indistinguishable from that of the wild-type NLG-1, suggesting that NRX-1 may not be essential or sufficient for NLG-1 clustering (Suppl. Fig. 1E).
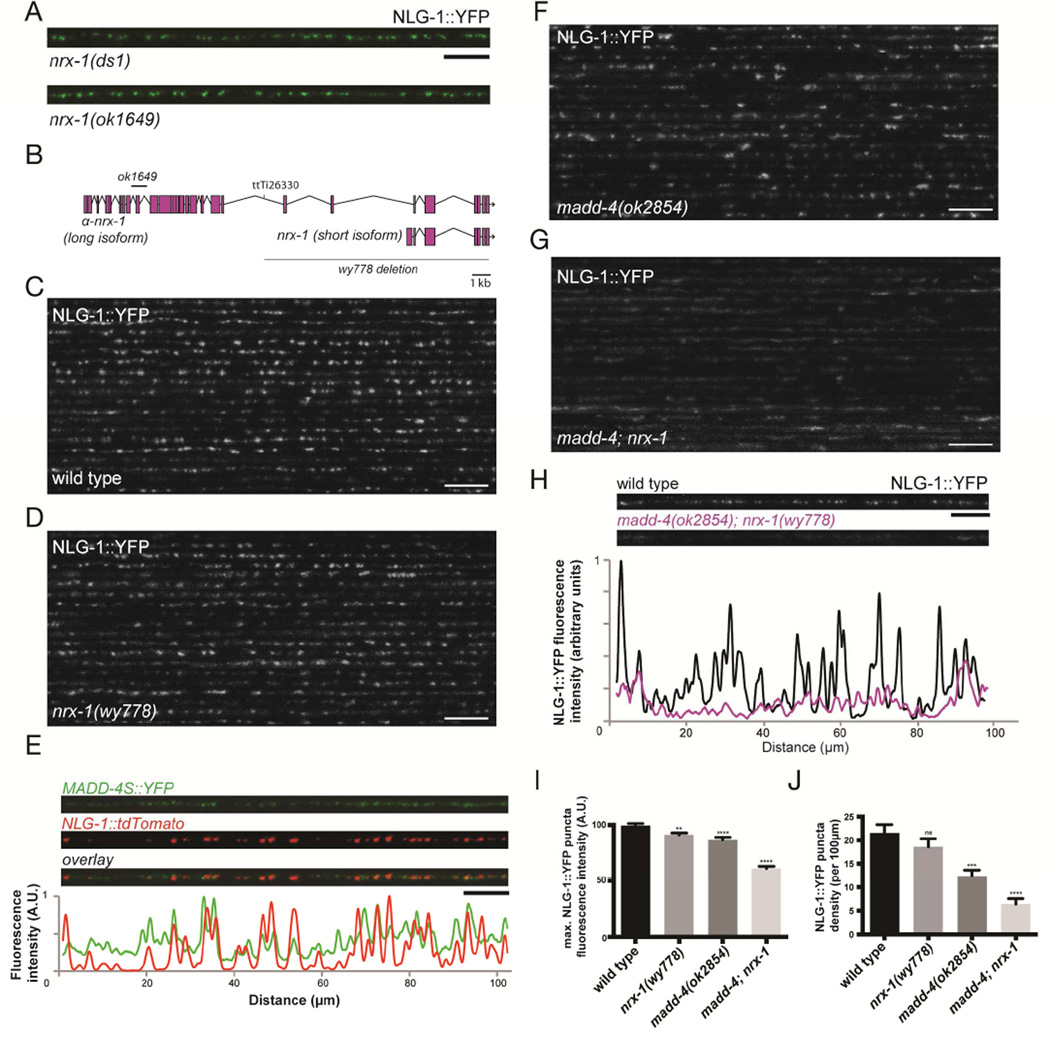
It remained possible that these mutations may not completely abolish binding between the worm NLG-1 and NRX-1 proteins. Therefore, we next analyzed various nrx-1 mutants. We further analyzed two deletion alleles in the long nrx-1 isoform, ds1 and ok1649. ok1649 has been reported to behave as a putative null for nrx-1 function (Calahorro and Ruiz-Rubio, 2013). Both alleles also failed to show any detectable NLG-1 clustering phenotype (Fig. 3A). But because both alleles only affected the long isoform of nrx-1, it was possible that the short form contributed to NLG-1 clustering. To further eliminate NRX-1 function, we generated a large deletion in the nrx-1 locus, nrx-1(wy778), which encompasses the transmembrane and cytoplasmic domains shared by all NRX-1 isoforms, and removes the short isoform entirely (Fig. 3B). In animals carrying this allele, we found no significant reduction in the clustering or intensity of NLG-1::YFP puncta (Fig. 3C–F), and no obvious defect in UNC-49 clustering (Fig. 4B, 4D and 4E), when compared to wild-type animals. Active zones and synaptic vesicles also seemed to accumulate normally at presynaptic terminals in the nrx-1(wy778) mutant (Suppl. Fig. 2). Therefore NRX-1 is likely dispensable for NLG-1 and GABAA receptors clustering. These results imply that NLG-1 becomes specifically associated with inhibitory postsynaptic sites through additional extracellular interactions.
Figure 3. MADD-4 and NRX-1 are redundantly required for NLG-1 localization at the synapse.
(A) Confocal micrographs of nrx-1(ds1) and nrx-1(ok1649) mutant animals showing NLG-1::YFP clusters in the dorsal nerve cord. (B) Schematic representation of the nrx-1 locus, showing the α-neurexin and β-neurexin isoforms, the Mos transposon insertion site (ttTi26330) as well as the ok1649 and wy778 deletions. (C,D) Confocal micrographs showing the dorsal nerve cord of 20 wild-type (C) and nrx-1(wy778) (D) animals expressing NLG-1::YFP. (E) Confocal micrographs of neuronallysecreted MADD-4S::YFP and muscle NLG-1::dtTomato at the dorsal nerve cord. The fluorescence profile of MADD-4S::YFP (green) and NLG-1::tdTomato (red), normalized to their respective maximum intensity, are shown. (F,G) Confocal micrographs showing the dorsal nerve cord of 20 madd-4(ok2854) (F) and madd-4(ok2854); nrx-1(wy778) double (G) mutant animals. (H) The fluorescence profiles of NLG-1::YFP in a wild-type and madd-4(ok2854); nrx-1(wy778) double mutant animal are shown. Both fluorescence profiles were normalized to the wild-type maximum intensity. (I,J) Quantification of NLG-1::YFP puncta maximum fluorescence intensity (I) and synaptic density (J). An ANOVA and post hoc Tukey’s tests were used to determine significant differences between the different genotypes. Error bars indicate SEM. p values are *<0.05, ***<0.001, ****<0.0001. Scale bars: 5 µm (A,E), 10 µm (C,D,F–H).
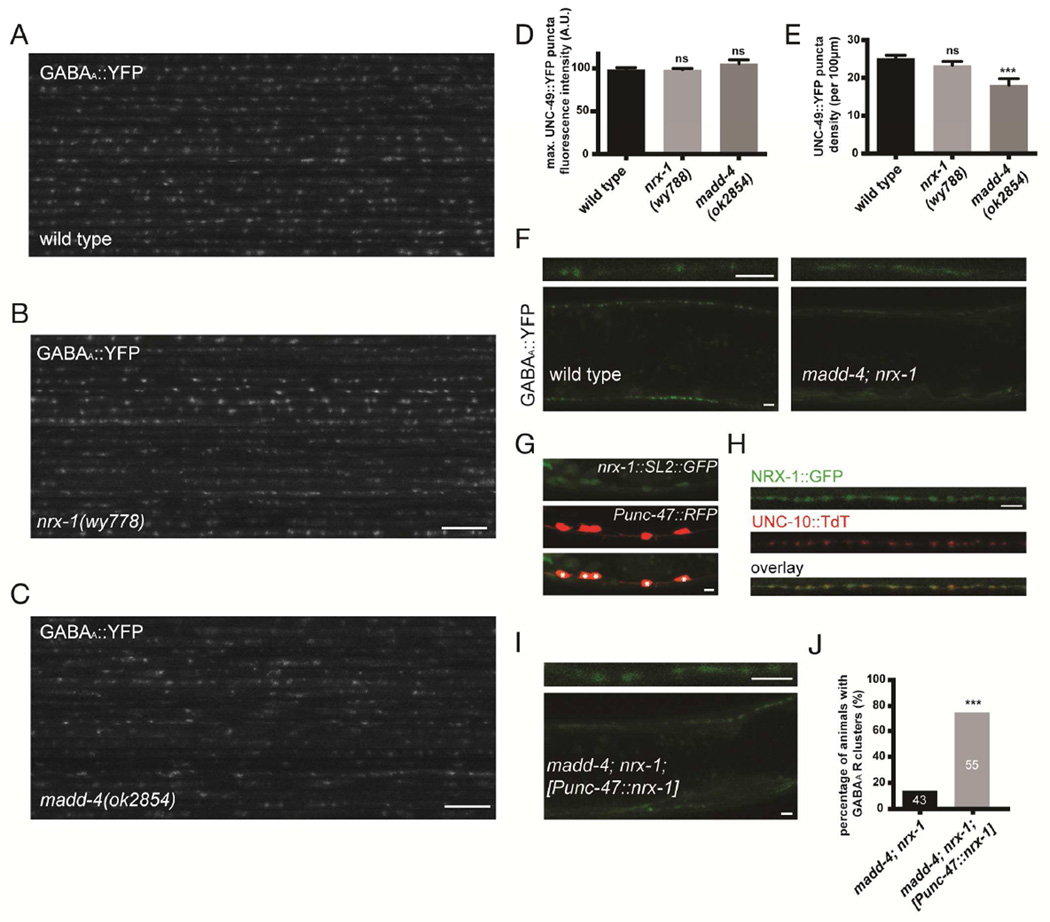
Figure 4. GABAA receptor clustering is impaired in the absence of MADD-4 and NRX-1.
(A–C) Confocal micrographs showing the dorsal nerve cord of 20 wild-type (A), nrx-1(wy778) (B) and madd-4(ok2854) (C) mutant animals. (D,E) Quantification of UNC-49::YFP puncta maximum fluorescence intensity (D) and synaptic density (E) in wild-type, nrx-1(wy778) and madd-4(ok2854) animals. An ANOVA and post hoc Tukey’s tests were used to determine significant differences between the different genotypes. Error bars indicate SEM. p values are ***<0.001. (F) Confocal micrographs showing the dorsal and ventral nerve cords of a wild-type and madd-4; nrx-1 double mutant animal expressing UNC-49::YFP. Top panels show a higher magnification of the dorsal nerve cord. (G) A transgene carrying a genomic fragment of nrx-1 fused to an SL2::GFP shows expression in GABAergic neurons, labeled in red (asterisks), as well as other ventral nerve cord neurons. (H) NRX-1::GFP localizes to inhibitory presynaptic sites, labeled with the active zone marker UNC-10::TdT. (I) Specific expression of a nrx-1 minigene in GABAergic neurons induces GABAAR clusters in madd-4; nrx-1 double mutants. (J) Quantification of GABAAR clustering in the madd-4; nrx-1 double mutant in the absence or presence of NRX-1 in GABAergic neurons. p<0.001, Fisher’s exact test. Scale bar: 10 µm (A–C), 3 µm (F–I).
NLG-1 and GABAAR clustering is compromised in the absence of Punctin/MADD-4 and NRX-1
A recent study showed that Punctin/MADD-4, an extracellular synaptic protein secreted from axonal terminals, is important for proper differentiation of cholinergic and GABAergic synapses (Pinan-Lucarré et al., 2014). The madd-4 locus encodes a long (MADD-4L) and short (MADD-4S) isoform from putative distinct promoters. While the long form is specifically localized to the cholinergic postsynapse, the short form is present at both cholinergic and GABAergic synapses (Pinan-Lucarré et al., 2014). Consistent with this study, we found that MADD-4S::YFP exhibited a punctate localization pattern along the nerve cord; a portion of the MADD-4S puncta colocalized with the NLG-1 clusters at GABAergic synapses (Fig. 3E). We then examined whether NLG-1 clustering was dependent on MADD-4, and found that NLG-1 puncta maximum fluorescence intensity was slightly reduced (−13%) compared to wild type, while synaptic density was reduced by 40% (Fig. 3F, 3I and 3J).
To test if MADD-4 and NRX-1 act redundantly to cluster NLG-1, we examined the madd-4; nrx-1 double mutant. In the absence of both genes, NLG-1::YFP becomes much more diffuse, when compared to the madd-4 single mutant (Fig. 3G). A line scan analysis of the NLG-1::YFP signal showed that peak intensities in the double mutant were dramatically reduced when compared to wild-type animals (Fig. 3H). We also found a dramatic decrease in synaptic density (−70%), as well as in the maximum synaptic fluorescence intensity (−38%) (Fig. 3I and 3J). These results suggest that MADD-4 and NRX-1 play critical, and largely redundant roles in clustering NLG-1 at postsynaptic specializations.
We next examined whether MADD-4 and NRX-1 are also required for GABAA receptor clustering at the postsynaptic terminal. Using the UNC-49::YFP marker, we found that maximum puncta fluorescence intensity was not changed, but synaptic density was reduced by 25% in madd-4 mutants (Fig. 4E), which is consistent with our observations with the NLG-1::YFP marker. Strikingly, UNC-49::YFP no longer formed any visible clusters in the madd-4(ok2854); nrx-1(wy778) double mutant (Fig. 4F), and appeared diffuse on the plasma membrane. Together, these results suggest that both NRX-1 and MADD-4 are responsible for recruiting NLG-1 and GABAA receptors to the postsynaptic terminals. Neurexin has been well characterized as a presynaptic protein in vertebrate systems. However, it has also been reported to function on the postsynaptic side of NMJs in C. elegans (Hu et al., 2012). To determine if NRX-1 could act in GABAergic neurons, we used two overlapping fosmids spanning the entire nrx-1 locus, and engineered a bicistronic GFP reporter at the 3’-end of nrx-1. The nrx-1::SL2::GFP transgene showed GFP expression in GABAergic neurons, as well as some other ventral cord motor neurons (Fig. 4G). Furthermore, a GFP-tagged NRX-1 expressed in GABAergic neurons localized to presynaptic sites (Fig. 4H). Finally, specific expression of a nrx-1 minigene in GABAergic motor neurons induced GABAAR clusters in madd-4; nrx-1 double mutants (Fig. 4I and 4J). These data suggest that NRX-1 acts at presynaptic sites, in trans- with NLG-1 on the muscle membrane, to cluster postsynaptic GABA receptors.
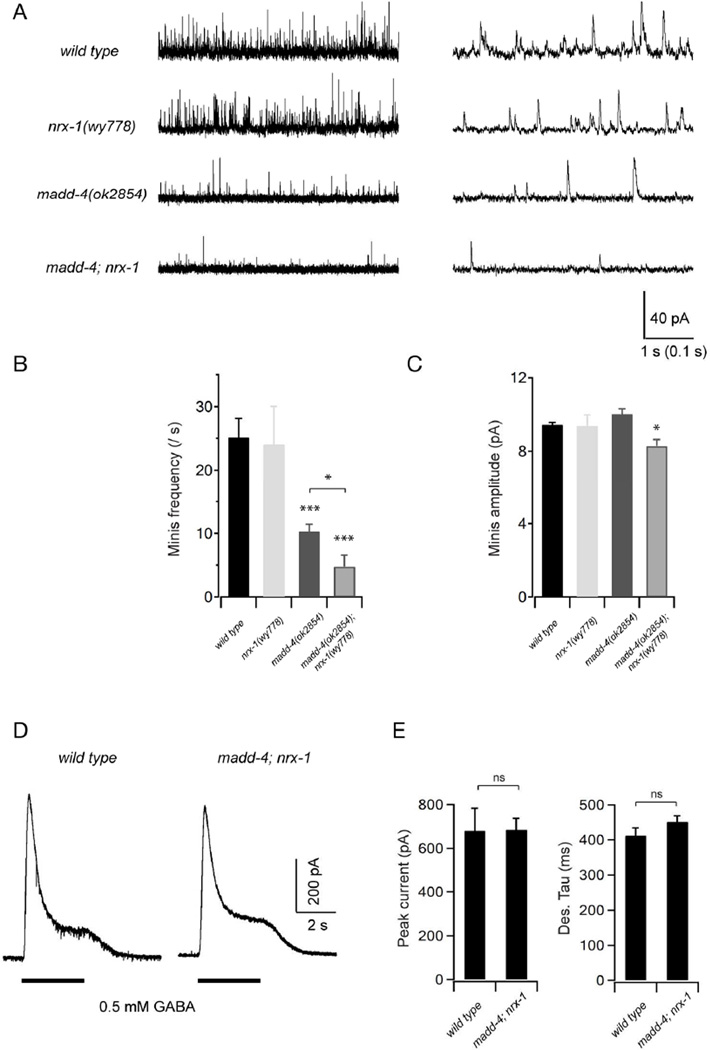
To further characterize the role of MADD-4 and NRX-1 at GABAergic synapses, we recorded spontaneous inhibitory postsynaptic currents (mIPSCs) in the muscles of the madd-4 and nrx-1 single, and the madd-4; nrx-1 double mutant animals (Fig. 5A). We found that both the frequency and amplitude of mIPSCs in nrx-1 single mutants were similar to that of wild-type animals (Fig. 5B and 5C). The frequency of mIPSCs was moderately reduced in the madd-4 single mutant, while the amplitude of mIPSCs was unchanged (Fig. 5B and 5C). In madd-4; nrx-1 double mutants, the mIPSC frequency was further and significantly reduced when compared to madd-4 single mutants (Fig. 5B). Importantly, the mIPSC amplitude was decreased in madd-4; nrx-1 double mutants (Fig. 5C), a phenotype shared by nlg-1 mutants. A reduced mIPSC frequency could result from either reduced presynaptic release, or decreased postsynaptic receptor numbers. Our NLG-1 and GABAA receptor clustering analyses (Fig. 3 and Fig. 4) as well as the muscle-specific requirement of NLG-1 to rescue GABAergic morphological defects (Fig. 1 and Fig. 2) point towards a functional contribution of reduced postsynaptic receptor density. To further assess if this is due to the dispersal or lack of the membrane GABA receptors in nrx-1; madd-4 mutants, we examined the evoked response in muscles upon exogenous GABA (0.5 mM) application. As was the case in the nlg-1 mutants, the amplitude and kinetics of GABA-evoked currents were unchanged in the madd-4; nrx-1 double mutants (Fig. 5D and 5E), suggesting that the total number of muscle membrane GABA receptors was unchanged. Together with our NLG-1 and GABAA receptor clustering analyses, these data strongly support the notion that MADD-4 acts together with NRX-1 to recruit NLG-1 and GABAA receptors to postsynaptic terminals.
Figure 5. MADD-4 and NRX-1 are redundantly required for GABAergic synaptic transmission.
(A) Representative traces of spontaneous GABAergic mIPSCs in wild-type (WT) animals, as well as nrx-1, madd-4 and madd-4; nrx-1 mutants with different time scales. Body wall muscles were held at −10mV. (B,C) Quantification of the frequency and amplitude of mIPSCs in various genetic backgrounds. Both the mIPSCs frequency and amplitude were significantly decreased in madd-4; nrx-1 double mutants, whereas only mIPSCs frequency was decreased in madd-4 single mutants. Neither mIPSCs frequency nor amplitude was significantly changed in nrx-1 mutants when compared to wild-type animals. (D) Representative traces of GABA (0.5 mM) evoked currents at muscle cells. (E) The peak amplitude and desensitization kinetics of GABA-evoked currents in madd-4; nrx-1 double mutants were comparable to that in wild-type animals. * p<0.05, *** p<0.001, ns: not significant, by the Mann-Whitney U test.
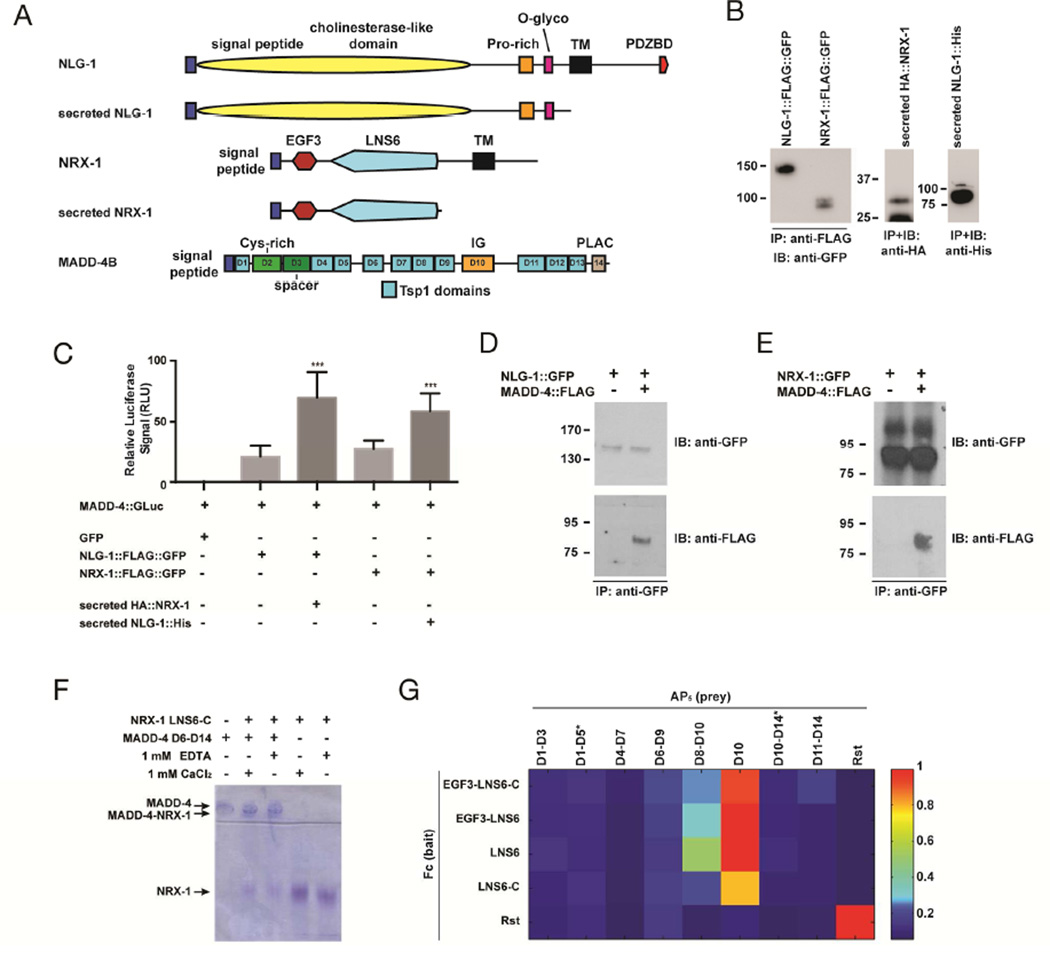
To further test this hypothesis, we assayed the biochemical interactions between these three molecules using several approaches. First, we used a luciferase cell-based assay previously used to probe the interaction between MADD-4S and various receptors (Chan et al., 2014). To ask whether MADD-4S could directly interact with NLG-1, we expressed luciferase-tagged MADD-4S from HEK293T cells, collected the conditioned media, and applied it to HEK293T cells transfected with NLG-1::FLAG::GFP. Upon immunoprecipitation of the NLG-1::FLAG::GFP receptors, we identified a higher luciferase activity in the washed immunoprecipitate, suggesting an interaction between the two proteins (Fig. 6C). Such an interaction was confirmed by co-immunoprecipitation experiments between secreted MADD-4S::FLAG and NLG-1::GFP transfected in HEK293 cells (Fig. 6D). Interestingly, using the luciferase assay, we found that the addition of secreted NRX-1[EGF3+LNS6] in the media potentiated the observed binding between NLG-1 and MADD-4S::GLuc (Fig. 6C), which raises the possibility that MADD-4S might also interact with NRX-1. We tested this hypothesis using the luciferase assay, and found increased levels of luciferase activity associated with cells expressing NRX-1::FLAG::GFP (Fig. 6C). Similar to our previous set of experiments, adding the extracellular domain of NLG-1 in the media increased the observed interaction between NRX-1 and MADD-4S (Fig. 6C). The direct interaction between NRX-1 and MADD-4S was confirmed by coimmunoprecipitation experiments (Fig. 6E).
Figure 6. MADD-4S interacts with NLG-1 and NRX-1.
(A) Schematic representations of NLG-1, NRX-1, and MADD-4S constructs used in binding assays. (B) Western blots showing immunoprecipitated proteins used for the luciferase binding assays. The Western blot on the left shows the transmembrane NLG-1 and NRX-1, the western blots on the right show the secreted NRX-1 and NLG-1 collected from culture media. (C) Normalized levels of MADD-4S::luciferase signal that immunoprecipitated with NLG-1::FLAG::GFP or NRX-1::FLAG::GFP in an HEK293T cell surface binding assay, in the absence or presence of secreted NRX-1 or NLG-1 ectodomains, respectively. An ANOVA and post hoc Tukey’s tests were used to determine significant differences between the different conditions. *** p<0.001. (D) HEK293T cells were transfected with NLG-1::GFP and incubated with concentrated conditioned media containing MADD-4S::FLAG. MADD-4S::FLAG was pulled down when NLG-1::GFP was immunoprecipitated. (E) HEK293T cells were transfected with NRX-1::GFP and incubated with concentrated conditioned media containing MADD-4S::FLAG. MADD-4S::FLAG was pulled down when NRX-1::GFP was immunoprecipitated. (F) Native PAGE shift assay for NRX-1 and MADD-4S. The NRX-1 construct used includes the LNS6 domain and the unstructured region to its C-terminus before the transmembrane helix (labeled as LNS6-C). NRX-1 and MADD-4S were used at 15 µM. (G) ECIA for various extracellular fragments of NRX-1 and MADD-4S. Binding is detected by absorbance at 650 nm. Drosophila Rst extracellular region was used as a negative control (against NRX-1 and MADD-4S), and a positive control as a hemophilic binder (lowest right corner). MADD-4S constructs with very poor expression are labeled with an asterisk (*), see Suppl. Fig. 3.
We applied native polyacrylamide gel shift assays using purified proteins to further dissect the physical interaction between MADD-4S and NRX-1. We created constructs of MADD-4S for expression in insect cells using baculoviruses and transient transfections. While we could not produce monodisperse full-length MADD-4S in appreciable quantities, we could produce parts of MADD-4S (Figure 6F). We observed that MADD-4S Domains 6 to 14 of MADD-4S binds to the LNS6 domain of NRX-1 using native polyacrylamide gel shifts (Fig. 6F). MADD-4S is a heavily positively-charged protein (pI: 8.8), and does not migrate in a native gel towards the anode (Fig. 6F, lane 1). Neurexin is an acidic protein (pI: 5.6) and migrates rapidly towards the anode (Fig. 6F, lanes 4 and 5). When mixed together at 15 µM, most of the free Neurexin and MADD-4S disappear and a new band appears representing the complex (pI: 8.4 assuming a 1:1 complex) (Fig. 6F, lanes 2 and 3). Complex formation is not dependent on the presence of Ca2+ or EDTA.
We further probed this binding using an avidity-based binding assay designed for extracellular proteins, called the Extracellular Interactome Assay (ECIA) (Özkan et al., 2014). Fc-tagged Neurexin (bait) produced in Drosophila S2 cells was captured on Protein A-coated microplates, which were incubated with Alkaline phosphatase-tagged, pentamerized MADD-4S fragments, as prey. Binding between bait and prey was detected using a colorigenic Alkaline Phosphatase substrate. Several MADD-4S constructs could produce detectable amounts of protein (Suppl. Fig. 3), and we could see that moderately expressing MADD-4S fragments of Domains 8 to 10 and Domain 10 (Immunoglobulin) alone interact with all NRX-1 fragments containing the LNS6 domain (Figure 6F). Hence, the native gel shift assays and the ECIA demonstrate a direct interaction between the LNS6 domain of NRX-1 and the Immunoglobulin domain of MADD-4S.
Together, our protein interaction data suggest a model whereby NRX-1 and MADD-4S cooperate to recruit NLG-1 through direct interactions within a NLG-1/MADD-4S/NRX-1 tripartite complex.
DISCUSSION
We set out to determine the localization and function of the C. elegans neuroligin ortholog at NMJs. Our results indicate that in muscles NLG-1 is specifically associated with inhibitory post-synapses, and that it is involved in the synaptic clustering of GABA receptors. Consistent with these findings, nlg-1 mutants show defects in spontaneous inhibitory postsynaptic currents.
Mammalian neuroligin family members NL1 and NL2 show preferential association with excitatory and inhibitory postsynapses, respectively (Song et al., 1999;,Varoqueaux et al., 2004). This raised the intriguing possibility that neuroligins might define the chemical subtype of specific synapses. We were interested in determining the localization pattern of the sole neuroligin familiy member in C. elegans, NLG-1. The NMJ represents a very suitable system to address this question since both excitatory cholinergic and inhibitory GABAergic postsynaptic sites are present in muscle cells, intercalate with one another along the dorsal and ventral nerve cords, and their position can be inferred from the localization of corresponding presynaptic sites. A generic postsynaptic localization could be suggestive of an ancestral role for neuroligin as a synaptic adhesion molecule that is diversified in higher organisms. In contrast we found that NLG-1 shows exquisite specificity for GABAergic postsynapses, and is absent from excitatory cholinergic postsynaptic sites. This suggests that the worm NLG-1 is functionally most analogous to the vertebrate NL2.
What could be the mechanisms that ensure specific localization of NLG-1 at inhibitory postsynapses? Mammalian NL2 has been shown to interact with two components of inhibitory postsynaptic scaffolds, gephyrin and collybistin (Poulopoulos et al., 2009). However the localization of NL2 is mostly unaltered in cerebellar Purkinje cells of GABAAα1 knockout mice (Patrizi et al., 2008), which subsequently lack gephyrin aggregates, or in collybistin knockout hippocampal neurons (Poulopoulos et al., 2009), which lack all three postsynaptic molecules, suggesting that NL2 clustering lies fairly upstream in the sequence of events leading to postsynaptic assembly. The situation in C. elegans is likely to be similar, since our results show that while NLG-1 is required for GABA receptor UNC-49 clustering, its own clustering is independent of UNC-49. In fact no collybistin ortholog is predicted from the C. elegans genome, and to the best of our knowledge, no synaptic function has been identified to date for the gephyrin ortholog moc-1, and paralog lin-46. Besides arguing against a role for postsynaptic scaffold molecules in determining NLG-1 localization, these observations also raise the question as to how NLG-1 recruits GABA receptors. Our analysis of truncated versions of NLG-1 suggests that proteins interacting with its PDZ-binding domain and its cytoplasmic tail might mediate this function.
Our structure-function analysis of NLG-1, together with the examination of various mutant alleles of nrx-1, strongly supported the idea that NLG-1 relied on extracellular cues other than NRX-1 for its localization. We found that Punctin/MADD-4, an extracellular synaptic protein secreted from axonal terminals implicated in synaptic differentiation (Pinan-Lucarré et al., 2014), plays important roles in clustering NLG-1 and GABAA receptors. Furthermore, three lines of evidences support the notion that Punctin/MADD-4 acts synergistically with NRX-1 to recruit NLG-1. First, while the nrx-1 mutant showed no defects in NLG-1 or GABAAR clustering, madd-4 mutants have a reduced number of clusters, a phenotype that is significantly enhanced in madd-4; nrx-1 double mutants. Second, our electrophysiological recordings showed a reduced frequency of miniature inhibitory postsynaptic current in madd-4 single mutant, which is also significantly enhanced in the madd-4; nrx-1 double mutant. Third, we found that MADD-4S directly interacts with both NLG-1 and NRX-1, and that the three molecules seem to bind to one another in a cooperative manner. Together, these pieces of evidence strongly argue that MADD-4 plays an essential role in clustering NLG-1 and GABAAR at postsynaptic specializations. Surprisingly, MADD-4’s function seems more important than NRX-1’s since little defects can be detected in the nrx-1 single mutant. The fact that madd-4; nrx-1 double mutant showed almost completely diffuse GABAAR and NLG-1 at the plasma membrane, as well as dramatically reduced mini IPSC indicate that these two proteins together account for the majority of postsynaptic organizing activities.
A companion paper by Tu et al. found similar roles for MADD-4S in recruiting NLG-1 to the postsynaptic sites of GABAergic synapses. They also report that MADD-4S binds to UNC-40/DCC, another receptor required for clustering GABAA receptors at inhibitory postsynaptic specializations. Together with the lack of obvious behavioral defects in nlg-1 mutants, these results indicate that they might be multiple parallel molecular pathways to recruit NLG-1 and postsynaptic receptors. Binding between MADD-4S and NRX-1 or UNC-40 argue that there might be crosstalk between some of these pathways.
Do similar mechanisms exist in other organisms? While a large body of literature supports the model that mammalian NLs and Neurexins are synaptic adhesion pairs that specify the function and development of various types of synapses, the genetic relationships between the various NLs and Neurexins are unclear due to the high level of redundancy in both gene families. This question has been best addressed in fruit flies. Loss of function of either dNlg1 or dNrx causes similar synaptic defects (Banovic et al., 2010). Interestingly, the dNlg1 mutant phenotypes are quantitatively stronger compared with the dNrx mutants, suggesting that factors such as Punctin/Madd-4 may play a role. Supporting this notion is the observation that dNrx binding is not absolutely required for DNlg1 function (Banovic et al., 2010). Whether the Drosophila Punctin is also necessary to cluster and activate dNlg1 remains to be tested. Another possible ligand for Neuroligin is thrombospodin, as suggested by an in vitro study (Xu et al., 2010).
A previous study showed that NRX-1 and NLG-1 are required for a retrograde signal that inhibit synaptic vesicle release at the cholinergic synapses (Hu et al., 2012). They found that NRX-1 functioned in the muscles while NLG-1 functions in the cholinergic neurons. The defect in retrograde signaling is only induced in the mir-1 mutant background. However, no synaptic release phenotype was reported in the single mutant phenotype. Hence, there is no obvious inconsistency between the role of NLG-1 at GABAergic postsynapses and its role at presynaptic terminals of cholinergic neurons.
We describe here a mechanism that clusters neurotransmitter receptors locally to achieve the precise apposition between pre- and postsynaptic specializations. MADD-4S is secreted from the inhibitory presynaptic terminal and deposited locally at the ECM near synapses. Together with the presynaptic adhesion molecule NRX-1, MADD-4S enriches NLG-1 at postsynaptic sites, which in turn clusters GABAA receptors (Suppl. Fig. 4). Hence, the GABA presynaptic terminals organize postsynaptic differentiation through the action of both synaptic adhesion molecules and secreted ECM proteins. Finally, this study suggests that the C. elegans NMJ could represent a powerful model system to identify new ligands for Neuroligin.
METHODS
Constructs and detailed cloning information are available upon request.
Strains & Genetics
Wild-type animals were of Bristol variety N2 strain. Strains were maintained using standard methods at 20°C.
Transgenic lines
wyEx5982 [Punc-47::mCherry::rab-3; Punc-129ΔBstEII::nlg-1::yfp; Podr-1::dsred], wyEx5983 [Punc-129::mCherry::rab-3; Punc-129ΔBstEII::nlg-1::yfp; Podr-1::dsred], wyEx5984 [Pmig-13::mCherry::rab-3; Punc-129ΔBstEII::nlg-1::yfp; Podr-1::dsred], wyEx4310 [Punc-129ΔBstEII::nlg-1; Podr-1::GFP], wyEx5284 [WRM0610dD09 nlg-1 fosmid; Podr-1::GFP], wyEx5333 [Phlh-1::nlg-1; Podr-1::GFP], wyEx5516 [Punc-47::nlg-1::CFP; odr-1::GFP], wyEx7279 [Punc-47::madd-4S::yfp; Punc-129ΔBstEII::nlg-1::tdTomato; Podr-1::gfp], wyEx4590 [Punc-129ΔBstEII::nlg-1::YFPΔextraC; Podr-1::GFP], wyEx4592 [Punc-129ΔBstEII::nlg-1::YFPΔPDZBD; Podr-1::GFP], wyEx4593 [Punc-129ΔBstEII::nlg-1::YFPΔintraC; Podr-1::GFP], wyEx7987 [Punc-129ΔBstEII::nlg-1ΔextraC; Podr-1::dsred], wyEx7988 [Punc-129ΔBstEII::nlg-1ΔPDZBD; Podr-1::dsred], wyEx7989 [Punc-129ΔBstEII::nlg-1ΔintraC; Podr-1::dsred], wyEx7990 [Punc-47::nrx-1; Podr-1::dsred], wyEx8021 [Punc-47::nrx-1::GFP; Punc-47::unc-10::TdT; Podr-1::GFP], krIs1 [Punc-47::SNB-1::CFP ; unc-49p::unc-49::YFP ; lin-15+] V, wyIs292 [Punc-47::unc-10::tdTomato; Punc-129ΔBstEII::nlg-1::yfp; Podr-1::dsred] III.
Mutants
LGI, madd-4(ok2854); LGIII, unc-119(ed3); unc-49(e382); LGV, ttTi26330; nrx-1(ds1); nrx-1(ok1649); nrx-1(wy778::unc-119+); LGX, nlg-1(ok259).
nrx-1 deletion mutant
We generated a large deletion in the nrx-1 locus using MosDel (Frøkjaer-Jensen et al., 2010). We obtained the IE26330 strain, which carries a Mos transposon insertion in an α-specific intron of nrx-1 (Fig. 4b), from the NEMAGENETAG consortium (Vallin et al., 2012). We designed a deletion cassette that contains an unc-119 rescuing construct, flanked by approximately 2 kb of genomic sequence upstream of the ttTi26330 insertion site, and approximately 3 kb of genomic sequence downstream of the nrx-1 STOP codon. The sequence encoding part of the extracellular domain of the long isoform is still present, but the deletion leads to a truncation of all α-isoforms before the transmembrane domain, and to an absence of the short isoform. The resulting deletion (wy778::unc-119+) spans a total of 12 kilobases, from the ttTi26330 insertion site to the STOP codon of the nrx-1 locus, is replaced with an unc-119 rescuing cassette, and was verified by long-range PCR using primers annealing outside of the deletion cassette.
Fluorescence microscopy
Images of fluorescently tagged fusion proteins were captured in live L4 C. elegans using a Plan-Apochromat 40 3 1.3 objective on a Zeiss LSM710 confocal microscope. Worms were immobilized using a mixture of 225 mM 2,3-butanedione monoxime (Sigma-Aldrich) and 2.5 mM levamisole (Sigma- Aldrich) in 10 mM Na HEPES. Confocal montages of dorsal nerve cords were assembled by imaging 20 L4 hermaphrodites of similar size in the mid-body region using identical image and laser settings for each genotype. Straightened dorsal cords were extracted from these images using the “straighten to line function” in the EMBL suite of ImageJ. For quantifying NLG-1::YFP intensity along dorsal nerve cords, background fluorescence was first subtracted by calculating the average intensity of each image in a region devoid of NLG-1::YFP puncta. Fluorescence intensities along the cord were calculated using the “plot profile” function of ImageJ, and the 95th percentile and 5th percentile were determined for each genotype. Synapse numbers was calculated using the “analyze particles” function, with an intensity threshold of 20 and a puncta size threshold of 10 pixels.
Electrophysiology
The dissection of C. elegans animals was described previously (Richmond and Jorgensen, 1999). Briefly, one-day old hermaphrodite adults were glued to a sylgard-coated cover glass covered with bath solution. The integrity of the anterior ventral body muscle and the ventral nerve cord were visually examined via DIC microscopy, and anterior muscle cells were patched using fire-polished 4−6 MΩ resistant borosilicate pipettes (World Precision Instruments, USA). Membrane currents were recorded in the whole-cell configuration by a Digidata 1440A and a MultiClamp 700A amplifier, using the Clampex 10 software and processed with Clampfit 10 (Axon Instruments, Molecular Devices, USA). Data were digitized at 10−20 kHz and Ziltered at 2.6 kHz.
The recording solutions were as described in our previous studies (Gao and Zhen, 2011). Specifically, the pipette solution contains (in mM): K-gluconate 115; KCl 25; CaCl2 0.1; MgCl2 5; BAPTA 1; HEPES 10; Na2ATP 5; Na2GTP 0.5; cAMP 0.5; cGMP 0.5, pH7.2 with KOH, ~320 mOsm. The bath solution consists of (in mM): NaCl 150; KCl 5; CaCl2 5; MgCl2 1; glucose 10; sucrose 5; HEPES 15, pH7.3 with NaOH, ~330 mOsm. Under these conditions, the reversal potentials are ~−30 mV and ~+20 mV for GABA and ACh receptors, respectively. To isolate mIPSC events, recordings were performed with a holding potential of −10mV, with 0.5 mM D-tubocurarine (d-TBC) included in the bath solution to block all acetylcholine receptors. γ-Aminobutyric acid (GABA, 0.5 mM) and control/wash solutions were puffed onto patched muscle cells using a perfusion system (VC-66CST Teflon Valve System, Warner Instruments) to obtain evoked GABA currents. All chemicals were from Sigma. Experiments were performed at room temperatures (20−22°C).
Statistic Analysis for electrophysiology
Two-tailed Mann-Whitney U test was used to compare the difference of the electrophysiological datasets. p<0.05 was considered statistically significant. Subsequent analysis and graphing were performed using Excel (Microsoft), Igor Pro (Wavemetrics) and Clampfit (Molecular Devices). In this study, N refers to the number of recordings (one cell per animal). All data are presented as mean ± SEM.
HEK293T cell surface binding experiments
The constructs used for cell transfections were pPRGS827 (pCDNA3-SS::HA::MADD-4S::Gaussia Luciferase (GLc), obtained from P. Roy(Chan et al., 2014)), pGM449 (pCMV::NLG-1::TM::FLAG::GFP), pGM451 (pCDNA3-SS::HA::NRX-1[EGF3+LNS6]), pGM467 (pCMV::NLG-1::His), pGM469 (pCDNA3-SS::HA::NRX-1[EGF3+LNS6]::TM::FLAG::GFP), pGM447 (pCMV::NLG-1::His::TM::GFP), pGM472 (pCMV::NLG-1::TM::GFP), pGM464 (pCDNA3-SS::HA::MADD-4S::FLAG).
Cell surface Gaussia Luciferase binding assays were performed as previously described (Chan et al., 2014). To obtain concentrated conditioned culture media containing HA::MADD-4S::Gluc or NRX-1[EGF3+LNS6], 10 µg of each respective expression plasmid was transfected into HEK293T cells on 10-cm dishes, and culture media was changed to DMEM−0.2% FBS. After 24 hours of culture, the conditioned media was collected and centrifuged to remove cellular debris, and subsequently concentrated 10 times using Pierce Protein concentrators 9K MWCO (Thermo Scientific). For the cell surface binding assays, 2 µg of plasmid expressing NLG-1::FLAG were transfected into HEK293T cells in 6-well plates. 48 hours posttransfection, culture media was removed and cells were incubated with concentrated conditioned media for 4 hours at 4°C. Cells were then harvested in a solution containing PBS, 0.2% BSA and a protease inhibitors cocktail (PIC, Sigma), and lysed in TNTE buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 10 mM NaF, 1 mM Na3VO4, PIC, 0.5% Triton X-100) containing protease inhibitors. NLG-1::FLAG and NRX-1::FLAG were immunoprecipitated using anti-FLAG EZview affinity gel (SIGMA), washed twice in lysis buffer, and then five times in TNTE buffer containing 0.1% Triton X- 100 plus protease inhibitor. Half of the immunoprecipitate was taken for Gaussia luciferase assays by using the BioLux Kit (NEB) and Synergy Neo Multi-Mode reader (BioTek). The other half was analyzed by Western blot for quantification of proteins.
For coimmunoprecipitation experiments, the same protocol was used with the following modifications. Receptors were transfected into HEK293T cells in 10-cm dishes. Secreted ligands were concentrated using VivaSpin protein concentrators 5K MWCO (VivaScience). 1 mg of protein from each reaction was immunoprecipitated with anti-GFP bound agarose beads (Chromotek) for 2 hours at 4°C. Blots were incubated with an anti-GFP antibody (Roche) to assess receptor levels and an anti-FLAG antibody (Sigma) to detect MADD-4S.
NRX-1/MADD-4S binding and gel-shift assays
Native gel shift assays were done using 4–15% PhastGels on a PhastSystem using native buffer strips (pH 8.8, GE Healthcare). Samples were run for approximately 200 accumulated Volt*hours. Proteins for this assay were expressed using baculovirus-infected High Five cells. ECIA was performed as explained in Özkan et al., 2014 with proteins produced in S2 cells using transient transfections.
Supplementary Material
Acknowledgements
Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). Mos mutant strains were generated by the Ewbank and Segalat labs as part of the NEMAGENETAG project funded by the European Community. They were distributed by M. Carre-Pierrat at the UMS 3421, supported by the CNRS. We thank Kevin Chan and Peter Roy for the pPRGS827 construct, as well as for advice on Luciferase assays. We also thank J.L. Bessereau for the krIs1 strain. This work is supported by the Canadian Institutes of Health Research (M.Z.), the Howard Hugues Medical Institute (K.S.) and the Stanford Institute for Neuro-Innovation and Translational Neurosciences (SINTN) (K.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
G.M. designed and performed genetics, cell biology and biochemistry experiments, collected and analyzed data, and wrote the manuscript. S.G. designed and performed electrophysiology experiments, and collected and analyzed data. A.O. performed the in vitro binding assays. W.H. performed biochemistry experiments and M.L. performed genetics experiments. E.O. and M.Z. helped design and oversee experiments. K.S. helped design, analyze, and oversee experiments and wrote the manuscript.
REFERENCES
- Araç D, Boucard AA, Ozkan E, Strop P, Newell E, Südhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56:992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Bamber BA, Beg AA, Twyman RE, Jorgensen EM. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J. Neurosci. 1999;19:5348–5359. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banovic D, Khorramshahi O, Owald D, Wichmann C, Riedt T, Fouquet W, Tian R, Sigrist SJ, Aberle H. Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron. 2010;66:724–738. doi: 10.1016/j.neuron.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Calahorro F, Ruiz-Rubio M. Human alpha- and beta-NRXN1 isoforms rescue behavioral impairments of Caenorhabditis elegans neurexin-deficient mutants. Genes Brain Behav. 2013;12:453–464. doi: 10.1111/gbb.12046. [DOI] [PubMed] [Google Scholar]
- Chan KKM, Seetharaman A, Bagg R, Selman G, Zhang Y, Kim J, Roy PJ. EVA-1 functions as an UNC-40 Co-receptor to enhance attraction to the MADD-4 guidance cue in Caenorhabditis elegans. PLoS Genet. 2014;10:e1004521. doi: 10.1371/journal.pgen.1004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat. Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C, Davis MW, Hollopeter G, Taylor J, Harris TW, Nix P, Lofgren R, Prestgard-Duke M, Bastiani M, Moerman DG, et al. Targeted gene deletions in C. elegans using transposon excision. Nat. Methods. 2010;7:451–453. doi: 10.1038/nmeth.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally C, Bessereau J-L. GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans. J. Neurosci. 2003;23:2591–2599. doi: 10.1523/JNEUROSCI.23-07-02591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Zhen M. Action potentials drive body wall muscle contractions in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2557–2562. doi: 10.1073/pnas.1012346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin S-X, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haklai-Topper L, Soutschek J, Sabanay H, Scheel J, Hobert O, Peles E. The neurexin superfamily of Caenorhabditis elegans. Gene Expr. Patterns. 2011;11:144–150. doi: 10.1016/j.gep.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Hu Z, Hom S, Kudze T, Tong X-J, Choi S, Aramuni G, Zhang W, Kaplan JM. Neurexin and neuroligin mediate retrograde synaptic inhibition in C. elegans. Science. 2012;337:980–984. doi: 10.1126/science.1224896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JW, Mullen GP, McManus JR, Heatherly JM, Duke A, Rand JB. Neuroligin-deficient mutants of C. elegans have sensory processing deficits and are hypersensitive to oxidative stress and mercury toxicity. Dis Model Mech. 2010;3:366–376. doi: 10.1242/dmm.003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Iida J, Hirabayashi S, Sato Y, Hata Y. Synaptic scaffolding molecule is involved in the synaptic clustering of neuroligin. Mol. Cell. Neurosci. 2004;27:497–508. doi: 10.1016/j.mcn.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Südhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993;364:334–337. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- Özkan E, Chia PH, Wang RR, Goriatcheva N, Borek D, Otwinowski Z, Walz T, Shen K, Garcia KC. Extracellular architecture of the SYG-1/SYG-2 adhesion complex instructs synaptogenesis. Cell. 2014;156:482–494. doi: 10.1016/j.cell.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi A, Scelfo B, Viltono L, Briatore F, Fukaya M, Watanabe M, Strata P, Varoqueaux F, Brose N, Fritschy J-M, et al. Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13151–13156. doi: 10.1073/pnas.0802390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinan-Lucarré B, Tu H, Pierron M, Cruceyra PI, Zhan H, Stigloher C, Richmond JE, Bessereau J-L. C. elegans Punctin specifies cholinergic versus GABAergic identity of postsynaptic domains. Nature. 2014 doi: 10.1038/nature13313. [DOI] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, Fowler SC, Malenka RC, Südhof TC. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron. 2000;26:345–356. doi: 10.1016/s0896-6273(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr. Opin. Neurobiol. 2011;21:132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin E, Gallagher J, Granger L, Martin E, Belougne J, Maurizio J, Duverger Y, Scaglione S, Borrel C, Cortier E, et al. A genome-wide collection of Mos1 transposon insertion mutants for the C. elegans research community. PLoS ONE. 2012;7:e30482. doi: 10.1371/journal.pone.0030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins Determine Synapse Maturation and Function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat. Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.