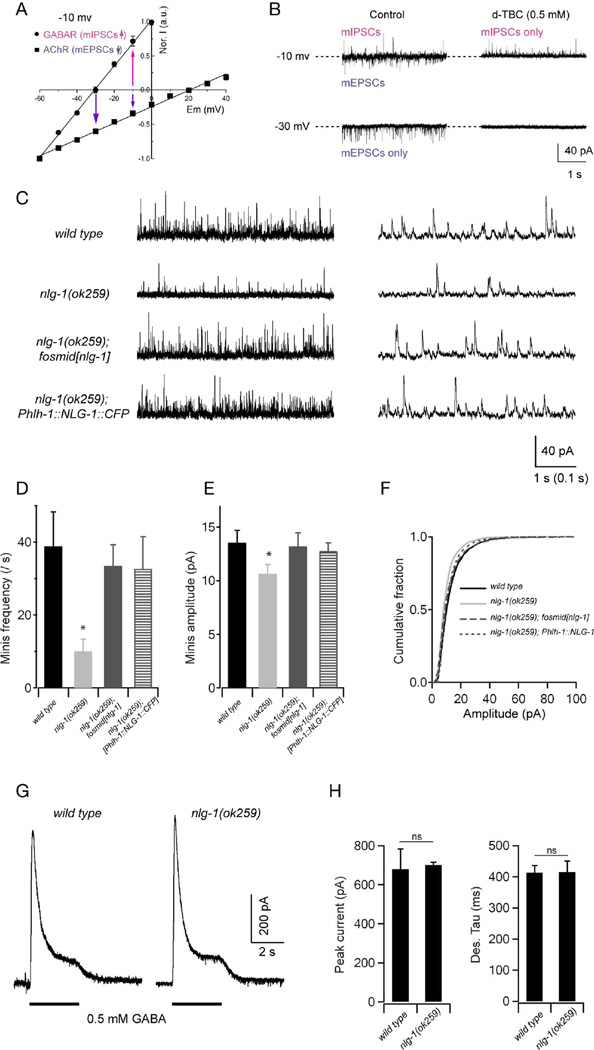
Figure 2. NLG-1 is required for normal GABAergic synaptic transmission.
Spontaneous inhibitory currents show reduced amplitude and frequency in nlg-1 mutants. (A,B) A protocol for recording GABAergic mIPSCs at the dissected muscle preparation. (A) In the recording solutions, the reversal potentials are around −30 mV and +20 mV for muscular GABAR and AChR, respectively. Adapted from Gao and Zhen, 2011. (B) Representative traces of spontaneous mIPSCs and mEPSCs in wild-type animals. When held at −10 mV, both mIPSCs and mEPSCs were recorded, as outward and inward currents, respectively. Application of 0.5 mM D-tubocurarine (d-TBC) allowed for the isolation of mIPSC events as it completely blocked mEPSCs. At −30 mV only mEPSCs were recorded (left bottom panel), since all events were blocked by 0.5 mM d-TBC (right bottom panel). (C) Representative traces with different time scales of spontaneous GABAergic mIPSCs in wild type, nlg-1(ok259), and transgenic nlg-1(ok259) mutants expressing NLG-1 under its endogenous promoter (nlg-1 fosmid), or a muscle-specific promoter (Phlh-1::NLG-1). Muscles were held at −10 mV. (D—F) Both the frequency and amplitude of mIPSCs were significantly decreased in nlg-1 mutants, and were restored to wild-type levels by expressing NLG-1 under its endogenous promoter, or a muscle-specific promoter. (G) Representative traces of GABA (0.5 mM) evoked currents at muscle cells. (H) The peak amplitude and desensitization kinetics of GABA-evoked currents in nlg-1 mutants were comparable to that in wild-type animals. * p<0.05, ns: not significant, by Mann-Whitney U test.