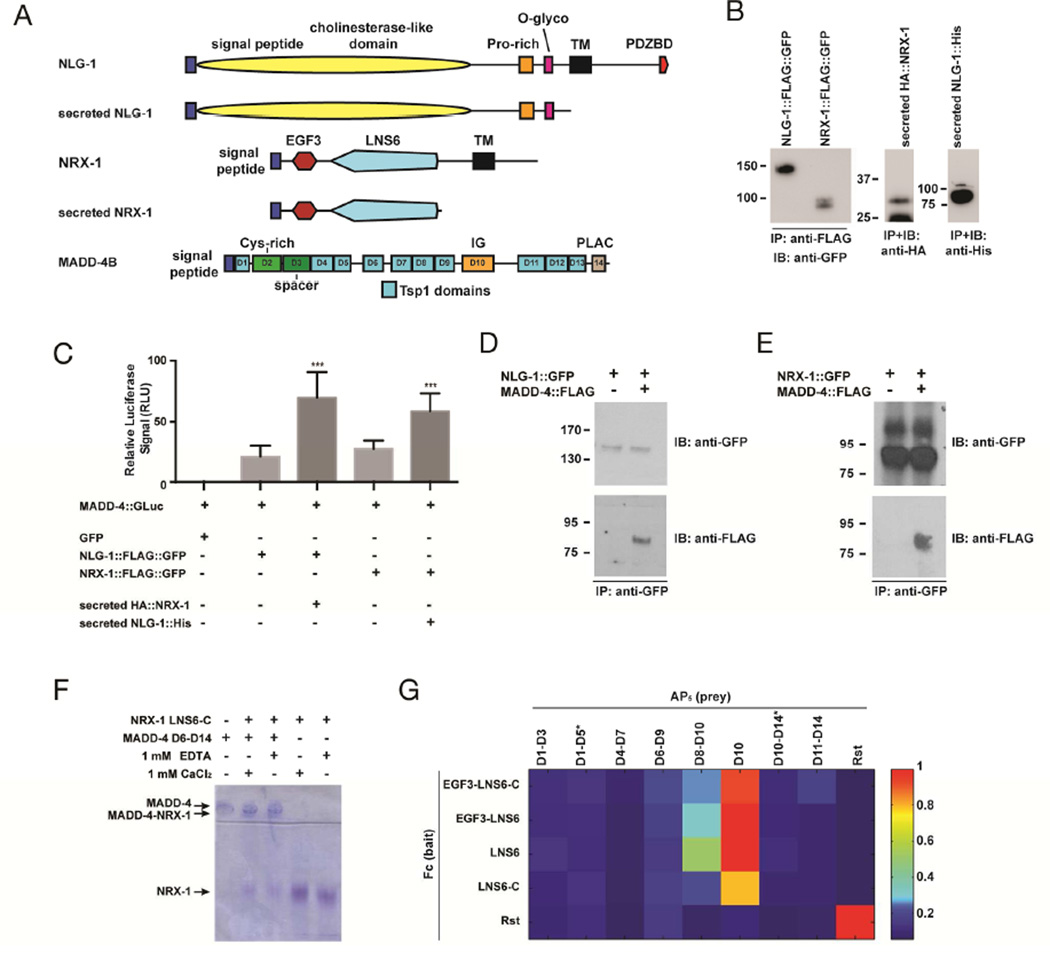
Figure 6. MADD-4S interacts with NLG-1 and NRX-1.
(A) Schematic representations of NLG-1, NRX-1, and MADD-4S constructs used in binding assays. (B) Western blots showing immunoprecipitated proteins used for the luciferase binding assays. The Western blot on the left shows the transmembrane NLG-1 and NRX-1, the western blots on the right show the secreted NRX-1 and NLG-1 collected from culture media. (C) Normalized levels of MADD-4S::luciferase signal that immunoprecipitated with NLG-1::FLAG::GFP or NRX-1::FLAG::GFP in an HEK293T cell surface binding assay, in the absence or presence of secreted NRX-1 or NLG-1 ectodomains, respectively. An ANOVA and post hoc Tukey’s tests were used to determine significant differences between the different conditions. *** p<0.001. (D) HEK293T cells were transfected with NLG-1::GFP and incubated with concentrated conditioned media containing MADD-4S::FLAG. MADD-4S::FLAG was pulled down when NLG-1::GFP was immunoprecipitated. (E) HEK293T cells were transfected with NRX-1::GFP and incubated with concentrated conditioned media containing MADD-4S::FLAG. MADD-4S::FLAG was pulled down when NRX-1::GFP was immunoprecipitated. (F) Native PAGE shift assay for NRX-1 and MADD-4S. The NRX-1 construct used includes the LNS6 domain and the unstructured region to its C-terminus before the transmembrane helix (labeled as LNS6-C). NRX-1 and MADD-4S were used at 15 µM. (G) ECIA for various extracellular fragments of NRX-1 and MADD-4S. Binding is detected by absorbance at 650 nm. Drosophila Rst extracellular region was used as a negative control (against NRX-1 and MADD-4S), and a positive control as a hemophilic binder (lowest right corner). MADD-4S constructs with very poor expression are labeled with an asterisk (*), see Suppl. Fig. 3.