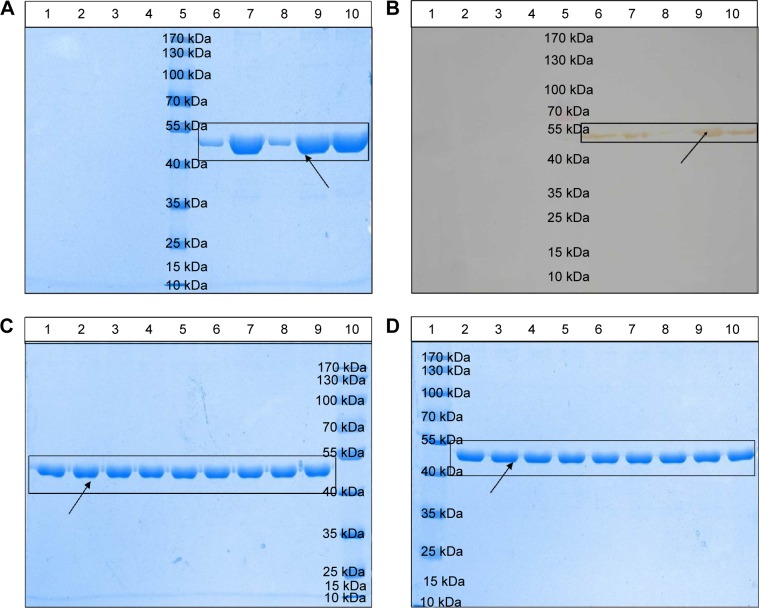
Figure 4.
Physical stability of the novel nanoemulsion vaccine.
Notes: (A) Primary structural integrity of the antigen protein. (B) Structural specificity of the antigen protein. (C) Primary structural integrity after storage at different temperatures. (D) Stability after long-term storage. Blank nanoemulsion supernatant: lane 1; blank nanoemulsion precipitate: lane 2; blank nanoemulsion treatment supernatant: lane 3; blank nanoemulsion treatment precipitate: lane 4; prestained marker: lane 5; novel nanoemulsion vaccine supernatant: lane 6; novel nanoemulsion vaccine precipitate: lane 7; novel nanoemulsion vaccine treatment supernatant: lane 8; novel nanoemulsion vaccine treatment precipitate: lane 9; native protein agent: lane 10, in (A) and (B). Lanes 1, 4, and 7 represent the novel nanoemulsion vaccine stored at 4°C for 1, 2, and 3 months individual; lanes 2, 5, and 8 represent novel nanoemulsion vaccine stored at 25°C for 1, 2, and 3 months individual; lanes 3, 6, and 9 represent novel nanoemulsion vaccine stored at 40°C for 1, 2, and 3 months individual, and lane 10 represents prestained protein marker, in (C). Lane 1: prestained marker; lanes 2–10 represent novel nanoemulsion vaccine stored at room temperature for 0, 1, 2, 3, 4, 6, 8, 10, and 12 months in (D). The visibly clear protein lanes were marked by black squares. Black arrows indicate antigen protein.