Fig. 4. PRMT8 is an HKD motif–dependent phospholipase.
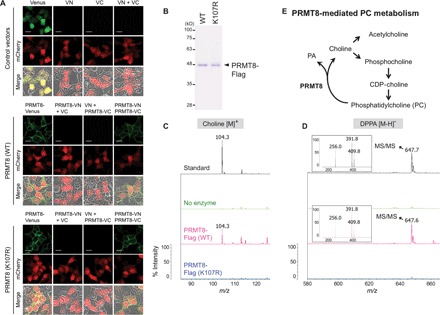
(A) BiFC experiments indicate dimerization of WT PRMT8 and the PRMT8 K107R mutant at the plasma membrane of HEK293T cells. All cells were cotransfected with BiFC pair vectors and mCherry vector as a transfection control. Scale bars, 20 μm. (B) Purified WT PRMT8 and the catalytically inactive PRMT8 K107R mutant were produced through baculoviral expression followed by Flag tag purification; proteins were visualized by Coomassie blue staining. (C and D) MS analysis of the reaction products generated by incubation of DPPC with either WT PRMT8-Flag or PRMT8 K107R mutant proteins. (C) Positive ion mode; (D) negative ion mode. Upper panels: Standard choline or DPPA with buffers but no enzyme. Middle panels: DPPC substrate with buffers but no enzyme. Bottom panels: DPPC substrate incubated with either WT PRMT8-Flag or PRMT8 K107R mutant proteins, resulting in the conversion of a portion of DPPC to the expected hydrolyzed products. Insets display the MS2 spectra of the following DPPA peaks at m/z 647.6: palmitic acid, m/z 256.0 [M − H]−; 16:0 lyso-PA, m/z 391.9 [M − H2O]−; 16:0 lyso-PA, m/z 409.8 [M − H]−. (E) Proposed working model for PRMT8-mediated PC metabolism.
