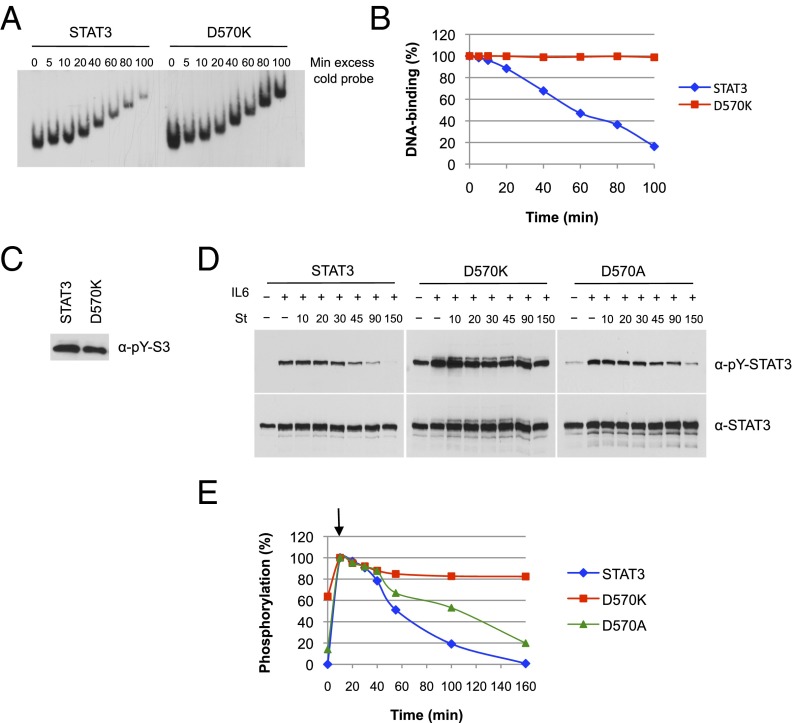
Fig. 4.
Activated D570K persistently binds to DNA and resists dephosphorylation. (A) DNA binding of D570K. Equal amounts of purified phospho-STAT3 and D570K were incubated for 15 min with 32P-labeled m67SIE probe. Excess of cold probe was then added at time 0, and the protein–DNA complexes were loaded onto the running gel at the indicated times. (B) Quantification of the data shown in A. (C) Purified phospho-STAT3 (4 ng) and D570K (4 ng) used in A were analyzed for Y705 phosphorylation (pY) by immunoblot. (D) Dephosphorylation kinetics of D570K and D570A. A4 cells were transfected with expression constructs for wild-type STAT3, D570K, or D570A and serum starved for 18 h. Parallel samples of cells were left untreated or treated with IL-6 (20 ng/mL) and soluble IL-6 receptor (25 ng/mL) for 10 min, and IL-6/IL-6 receptor for 10 min followed by the tyrosine kinase inhibitor staurosporine (St, 1 μM) for the indicated times (10, 20, 30, 45, 90, and 150 min). Nuclear extracts were prepared and analyzed by Western blotting for phospho-Y705 STAT3 (Top) and total STAT3 (Bottom). (E) Phospho-STAT3 signals were quantified by ImageJ software and normalized for total STAT3 signals. The graph represents the experiment shown in (D). Arrow denotes start of staurosporine treatment after 10 min of IL-6.