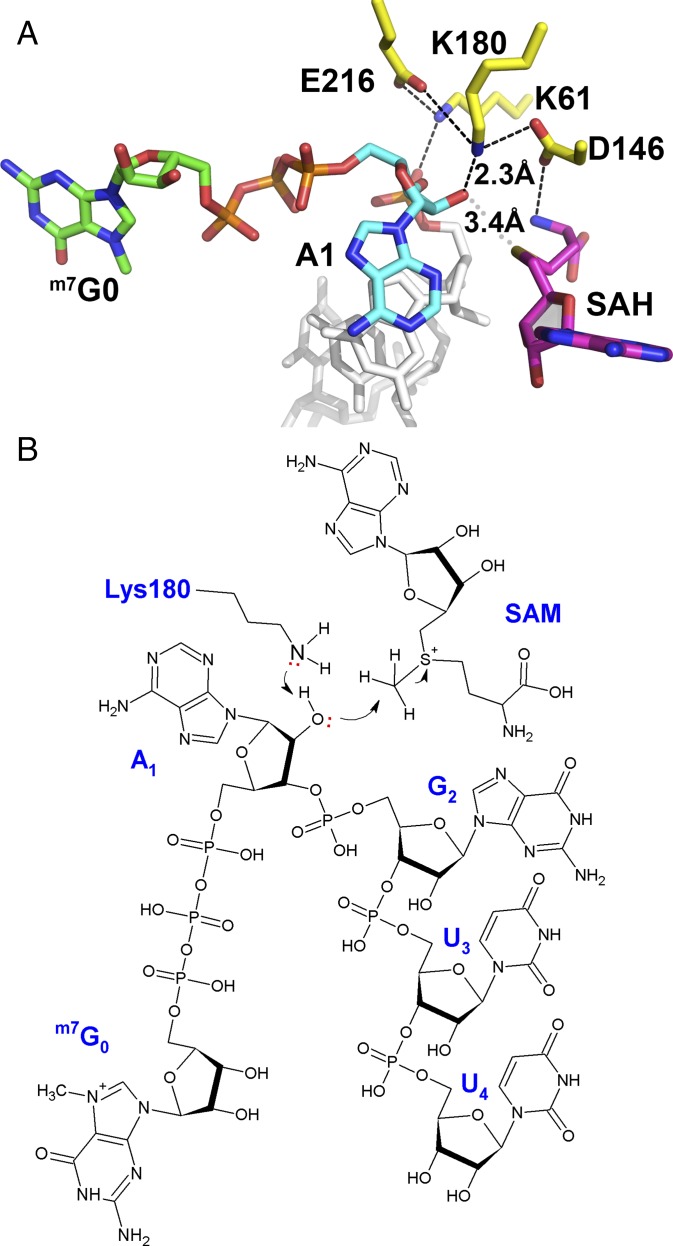
Fig. 2.
Proposed enzymatic mechanism for 2′-O-methylation by the flavivirus NS5 MTase. (A) Close-up view of the MTase active site with the K61-D146-K180-E216 catalytic tetrad shown as yellow sticks and SAH in magenta sticks. Color code for the bound RNA: G0 carbon atoms, green; A1, cyan; G2U3U4, gray. The close contacts between the 2′-oxygen atom of adenine A1, the amino group of K180, and the sulfur group of SAH are indicated by dashed lines, and the corresponding distances are given. (B) Schematic view of an active ternary complex comprising cap-0 RNA and the methyl donor SAM, based on the present structure. The stereochemistry of the reactants (distances and angles) conforms to what is expected from an inline SN2 reaction.