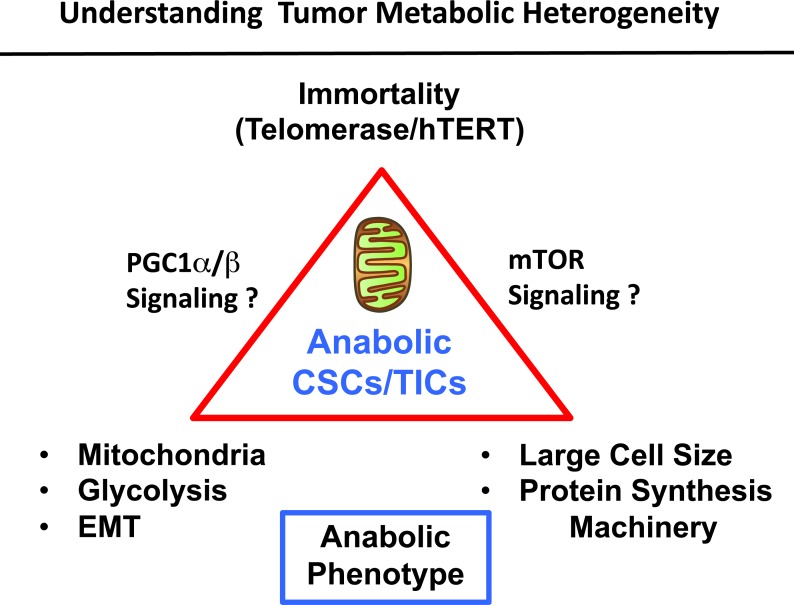
Figure 7. Understanding the role of immortality, anabolic metabolism and cell size in stem-like cancer cells.
Here, we used FACS analysis to begin to dissect metabolic heterogeneity in tumor cells. More specifically, we showed that anabolic stem-like cancer cells can be purified using hTERT-eGFP as a surrogate marker of telomerase activity. These eGFP-high cells showed increased stem cell activity (mammosphere formation), as well as functional increases in mitochondrial mass and activity. Further studies with unbiased label-free proteomics revealed the upregulation of mitochondrial proteins, glycolytic enzymes and EMT markers, as well as ribosome subunits and other components of the proteins synthesis machinery. These proteomic studies are consistent with, and support an anabolic stem-like cancer cell phenotype. Quantitatively similar results were obtained using large cell size to purify anabolic stem-like cancer cells. We speculate that high telomerase activity drives an increase in mitochondrial power, via positive regulation by PGC1-α/β. Moreover, we suggest that increased mTOR signaling may contribute to larger cell size, via increased protein synthesis. The PI3K/AKT/mTOR pathway is known to i) control cell size by positively regulating protein synthesis and ii) telomerase (hTERT) forms a physical complex with mTOR and can therefore regulate its activity. In summary, our results metabolically define a sub-population of stem-like, mitochondrial-rich, cancer cells, allowing us to understand the possible origins of metabolic heterogeneity in human tumors.