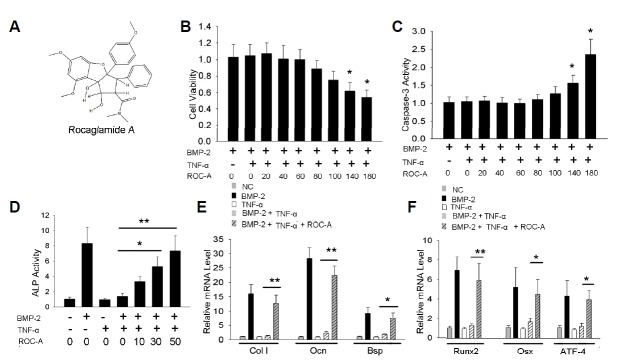
Fig. 1.
Rocaglamide-A prevented the TNF-α induced inhibition of osteoblast differentiation in C2C12 cells. (A) The chemical structure of rocaglamide-A (ROC-A). (B, C) The MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] assay was used to test the cell viability. The results revealed that concentrations of 20 to 100 nM ROC-A caused no significant decrease in cell viability; however, concentrations of 140 or 180 nM ROC-A led to a notable decrease of cell viability (n > 3, *p < 0.05). Concentrations of 20 to 100 nM ROC-A caused no significant elevation of caspase-3 activity; however, 140 or 180 nM ROC-A treatment led to a notable increase of caspase-3 activity (n > 3, *p < 0.05, **p < 0.01) (D) Concentrations of 30 or 50 nM ROC-A rescued the suppression of alkaline phosphatase (ALP) activity from 10 ng/ml tumor necrosis factor-α (TNF-α): the cells treated with 50 nM ROC-A exhibited a 7.3 ± 1.9-fold promotion of ALP activity compared to the control group (n > 3, **p < 0.01). (E) At a concentration of 50 nM, ROC-A significantly protected the gene expression of type I collagen (ColI), osteocalcin (Ocn) and bone sialoprotein (Bsp) from the suppression of 10 ng/ml TNF-α (n > 3, *p < 0.05, **p < 0.01). (F) At a concentration of 50 nM, ROC-A significantly protected the gene expression of runt-related transcription factor 2 (Runx2), osterix (Osx), and activating transcription factor 4 (ATF4) from the suppression of 10 ng/mL TNF-α (n > 3, *p < 0.05, **p < 0.01).