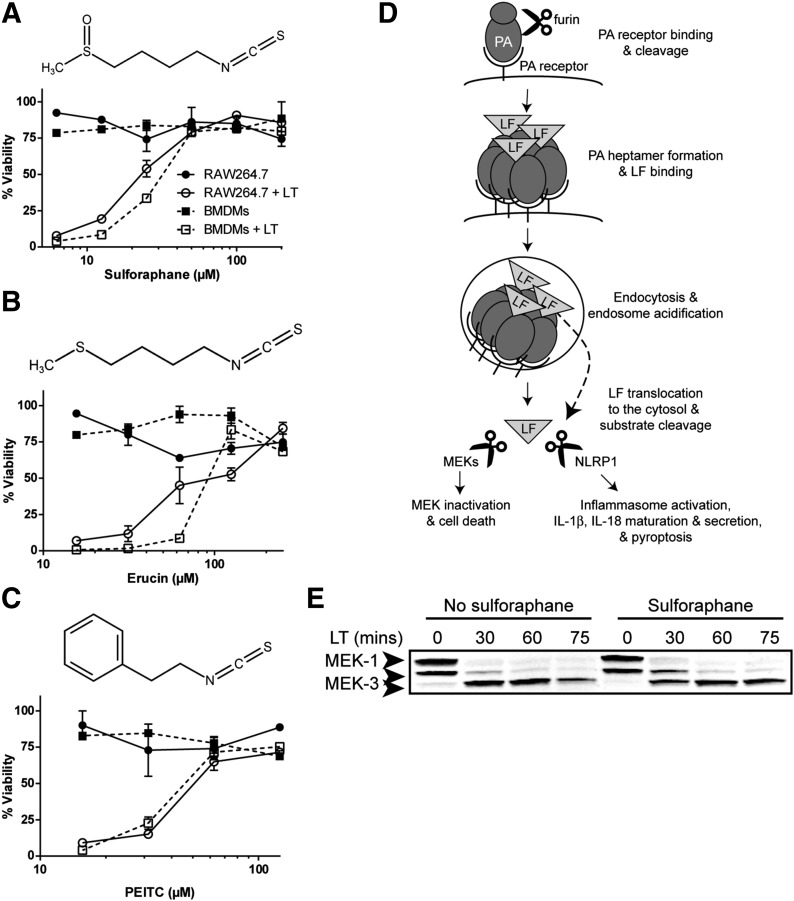
Figure 1. SFN inhibits LT-induced pyroptosis but does not inhibit LF release into the cytosol or protease activity.
(A–C, top) Chemical structure of SFN (A), erucin (B), and PEITC (C). (A–C, bottom) RAW264.7 or primary Balb/cJ BMDM cells were treated with or without SFN (A) for 30 min or erucin (B) or PEITC (C) for 1 h over a range of concentrations. Cells were then treated with or without LT (1 μg/ml) for 2 h, and viability was assessed by MTT staining. Data are means ± sem of 2 biologic replicates and are representative of 2 or more independent experiments. Legend is shown in (A). (D) Steps required in macrophage entry and substrate cleavage by B. anthracis LF: PA binds its receptor, is cleaved by the furin protease, and oligomerizes to form a heptamer. LF binds the PA heptamer, the complex is endocytosed, and endosome acidification triggers PA pore formation and LF translocation to the cytosol. LF cleaves its substrates, the MEKs, initiating cell-cycle arrest and apoptosis, and NLRP1 (in select rodent strains), leading to caspase-1 recruitment and activation, IL-1β and IL-18 maturation and secretion, and pyroptosis (modified from Liu et al. [40]). LF translocation to the cytosol and protease activity can be monitored by MEK cleavage. (E) Balb/cJ BMDM cells were treated with or without SFN (50 μM) for 30 min then with or without LT (1 μg/ml) for 30, 60, or 75 min and MEK1 and MEK3 cleavage in cell lysates was assessed by Western blot. The N-terminal epitope recognized by the anti-MEK1 Ab is lost upon LF cleavage. The N-terminal epitope recognized by the anti-MEK3 Ab is not lost, and cleavage is observed by a change in mobility. The same gel that was probed for MEK1 was probed for MEK3, demonstrating equal protein loading. Data are representative of 2 independent experiments.