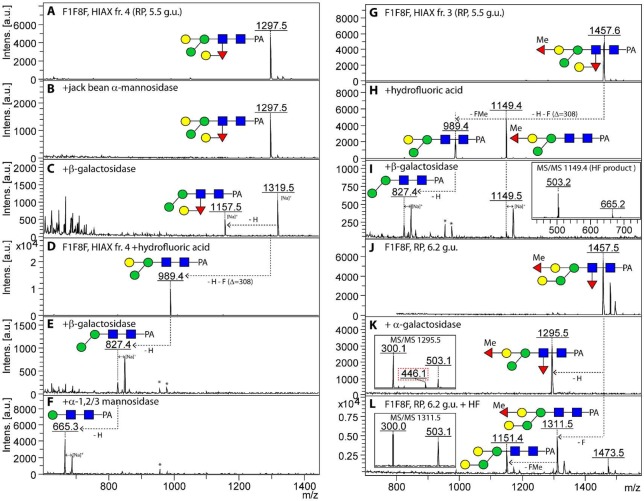
Figure 5.
Enzymatic and chemical treatments of Hex4HexNAc2Fuc1–2Me0–1 glycans from the fut-1;fut-8 strain. The 2D-HPLC fraction (HIAX fraction 4 of the 5.5 g.u. RP-HPLC fraction; Figure 4A, MS/MS spectrum of m/z 1297 is shown in Figure 2C) was treated sequentially with (B,C) jack bean α-mannosidase and then Aspergillus β-galactosidase or with (D–F) hydrofluoric acid, followed by Aspergillus β-galactosidase and finally α1,2/3-mannosidase. For the two variants of m/z 1457 ((G) HIAX fraction 3 of the 5.5 g.u. RP-HPLC fraction and (J) the 6.2 g.u. RP-HPLC fraction), a differential effect of hydrofluoric acid was observed (pairs of products of m/z 989/1149 or 1151/1311, which are due to losses of 146, 160, or 308 Da (H,L)); the effects of subsequent treatment of the former with Aspergillus β-galactosidase (I) or digestion of the latter solely with α-galactosidase (K) also indicated their structural difference. The absence of the putative initial rearrangement fragments of low intensities at m/z 608 or 446 for these glycans (for MS/MS see Figure 2G,H) only after hydrofluoric acid treatment (insets in panels I and L) is consistent with a correlation between the presence of these rearrangement ions and distal core fucosylation. Note that the properties of the distal GalFuc modification contrast in terms of β-galactosidase and hydrofluoric acid sensitivity with the reducing-terminal Galβ1,4Fucα1,6 motif. (See data on glycans from the fut-1;fut-6 strain.) Components in the β-galactosidase preparation resulted in a shift to sodiated adducts. Asterisks indicate nonglycan contaminants in the enzyme preparations.