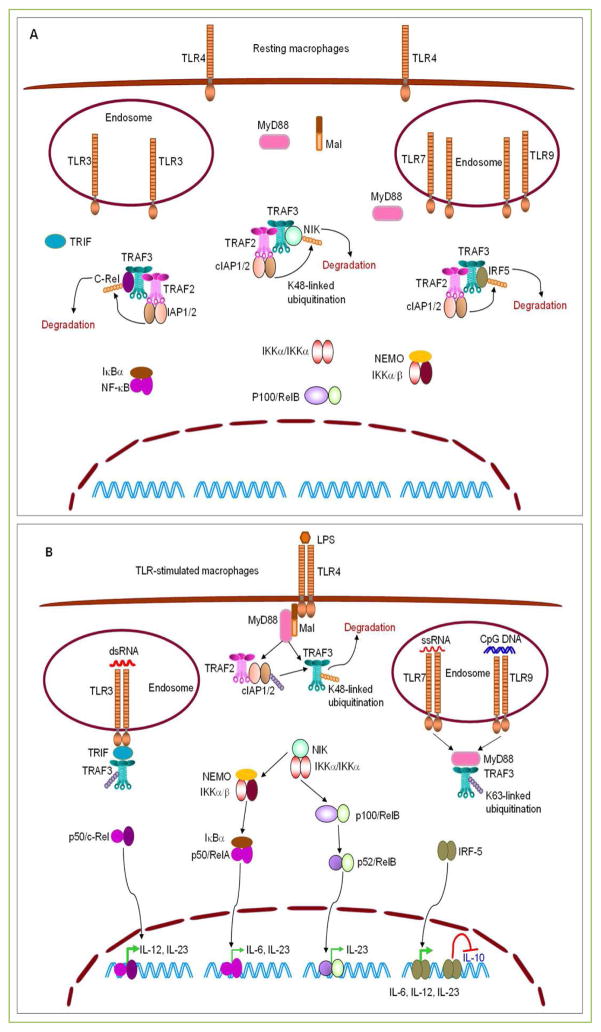
Figure 1. TRAF3-mediated inhibition of proinflammatory cytokine production induced by TLRs in macrophages.
(A) TRAF3 targets NIK, c-Rel and IRF5 for degradation in resting macrophages. In the absence of stimulation, TRAF3 constitutively binds to NIK, c-Rel, and IRF5, and bring them to the TRAF3-TRAF2-cIAP1/2 complexes. In these complexes, the E3 ligases cIAP1/2 catalyze the K48-linked ubiquitination on NIK, c-Rel, and IRF5, thereby targeting them for proteasome-mediated degradation. Thus, TRAF3 prevents the activation of NF-κB2, NF-κB1, and IRF5 in resting macrophages. (B) TRAF3 inhibits TLR-induced proinflammatory cytokine production in macrophages. In response to LPS stimulation, dimerized or oligomerized TLR4 recruits Mal and MyD88, which in turn recruits TRAF3 and the associated TRAF2-cIAP1/2 complex to the receptor signaling complex at the plasma membrane. This allows the activation of cIAP1/2 to target TRAF3 for K48-linked ubiquitination and degradation. Similarly, stimulation of TLR7 by ssRNA or stimulation of TLR9 by CpG DNA also recruits TRAF3 via MyD88, while engagement of TLR3 by dsRNA recruits TRAF3 via TRIF to the receptor signaling complexes at the endosome membrane. Recruitment of TRAF3 by dimerized or oligomerized TLRs via MyD88 or TRIF disrupts the interaction between TRAF3 and NIK, c-Rel, or IRF5. This results in the accumulation of NIK, c-Rel and IRF5, and subsequent activation of NF-κB2 (p52/RelB), p50/c-Rel, and IRF5, which promote the expression of the proinflammatory cytokines IL-6, IL-12, and IL-23 in stimulated macrophages. In addition, nuclear IRF5 also inhibits the expression of the anti-inflammatory cytokine IL-10. Ablation of TRAF3 from macrophages mimics TLR engagement and also releases NIK, c-Rel and IRF5 from the TRAF2-cIAP1/2 complexes, allowing the accumulation of NIK, c-Rel and IRF5. Therefore, TLR agonists induce enhanced production of the proinflammatory cytokines IL-6, IL-12 and IL-23 but decreased production of the anti-inflammatory cytokine IL-10 in TRAF3−/− macrophages. Transcription factors (p50/c-Rel and IRF5) that play major roles in driving the production of IL-6, IL-12 and IL-23 are shown in bold green arrows. TRAF3-independent TLR signaling pathways, including TRAF6- or TRAF2-induced activation of ERK1/2, p38, JNK1/2 and NF-κB1 (p50/RelA), are not depicted in the figures.