Abstract
Objective:
To evaluate the efficacy and safety of canagliflozin in combination therapy among patients with type 2 diabetes mellitus with inadequate glycemic control.
Methods:
Two review authors independently searched for the relevant randomized controlled clinical trials from the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, IndMed, LILACS, and clinical trials registry www.clinicaltrials.gov. Primary outcomes for this review included: change in hemoglobin A1c (HbA1c) levels, fasting plasma glucose (FPG) levels and risk of occurrence of genital mycotic infections at 26 weeks. We combined results using mean difference (MD) for continuous data, and risk ratio (RR) for dichotomous data.
Results:
Of the 124 identified reports, five RCTs with 3565 participants were eligible for the meta-analysis. All included studies had compared canagliflozin 100 mg and 300 mg once daily with placebo or sitagliptin 100 mg once daily. We judged that most of the studies had low risk of bias or unclear risk of bias in five major domains. Canagliflozin 300 mg once daily led to a significant decrease in HbA1c levels (IV Fixed -0.77, 95% CI [-0.90, -0.64] P < 0.00001) and FPG levels (IV Fixed -2.08; 95% CI [-2.32, -1.84], P <0.00001), body weight, systolic blood pressure and triglyceride levels after 26 weeks as compared to placebo. There was a also a significant difference in the efficacy of canagliflozin 300 mg and sitagliptin 100 mg once daily in favour of canagliflozin. Both doses of canagliflozin led to genital mycotic infections among males and females, urinary tract infections, pollakiuria, polyuria and postural dizziness.
Conclusions:
Canagliflozin significantly decreases HbA1c and FPG levels and body weight as compared to placebo among patients with inadequate glycemic control with an earlier regime of glucose lowering agents. Long term safety studies are required to evaluate the incidence of adverse events.
Keywords: Canagliflozin, genital infections, hemoglobin A1c, type 2 diabetes mellitus
INTRODUCTION
The Global Diabetes Atlas 2014 shows that 387 million people have diabetes mellitus and the incidence of type 2 diabetes mellitus (T2DM) is rising across the world.[1] About 77% of the patients with diabetes live in low and middle income countries. The Western Pacific area has 138 million patients, which is the maximum in a region over world.[1] The epidemiologic picture is better in the Untied States as compared to the world, as there is a steady improvement in the proportion of patients with T2DM achieving the target hemoglobin A1c (HbA1c) levels, blood pressure, and low density lipoprotein (LDL-C) levels in the last 10 years.[2] Still, 33–49% of patients are not able to achieve adequate glycemic control, blood pressure, and cholesterol control, and just 14% are able to achieve all three targets and a nonsmoking status.[2]
Diabetes mellitus is a chronic progressive disease, requiring multipronged continuous care for optimal glycemic control, prevention of acute complications, and decrease in the risk of chronic complications such as retinopathy, neuropathy, nephropathy, and cardiovascular diseases.[2,3] Currently, a wide variety of treatment modalities targeting different pathologic processes are available. According to the American Diabetes Association (ADA), metformin is the preferredfirst drug of choice with lifestyle modifications for patients with T2DM.[4] In addition to metformin, other available glucose-lowering agents include insulin secretagogues (sulfonylureas, meglitinides [repaglinide and nateglinide] glucagon-like peptide-1 [GLP-1] agonist and dipeptidyl peptidase-4 inhibitors); insulin sensitizers (thiazolidinediones); alpha glucosidase inhibitors; and various insulin formulations.[3,4] The ADA 2015 guidelines recommend the addition of any other treatment regimen to metformin if the glycemic control is not achieved after approximately 3 months of the start of treatment.[2]
Most importantly, the pharmacotherapy of T2DM should be patient-centered, considering aspects such as efficacy, adverse effects, and cost, at the time of decision.[2] In addition, both the healthcare professional and the patient prefer and adhere to those regimes which are more patient-centered, more convenient with less adverse effects, and help in achieving adequate glycemic control. The currently available drugs have their own specific uses and adverse effects, thus restricting their use, for example, sulfonylureas are known to cause weight gain, hypoglycemia, and secondary failure; meglitinides also cause secondary failure, hypoglycemia, and are most effective for postprandial hyperglycemia; GLP-1 agonists have to be given subcutaneously and commonly cause nausea and vomiting; thiazolidinediones (pioglitazone and rosiglitazone) may lead to weight gain, edema, congestive heart failure, and increase the risk of cardiovascular diseases.[3] In this scenario, another drug group has been added to the armamentarium of glucose-lowering agents available for treatment of T2DM. Canagliflozin, a sodium glucose co-transporter (SGLT-2) inhibitor, was approved by the US Food and Drug Administration (US FDA) for use in the management of T2DM.[5] SGLT2 is mainly responsible for renal glucose reabsorption. Usually, almost all filtered glucose is reabsorbed from the renal tubules till the filtered load exceeds the glucose reabsorptive capacity and then urinary excretion of glucose starts.[6,7] This level is referred as the renal threshold for glucose (RTG). The RTG level is raised among patients with T2DM. Canagliflozin lowers the threshold level by inhibiting the renal transporters (SGLT2).[5,8] This results in increased urinary glucose excretion, mild osmotic diuresis, and increased caloric loss with a minimal risk of hypoglycemia through an insulin-independent mechanism.[5,8]
Canagliflozin and other drugs of the same group, dapagliflozin and empagliflozin, are approved for use as monotherapy or as part of a combination regime for patients with T2DM. The efficacy of canagliflozin has been demonstrated as compared to placebo and active comparators such as sitagliptin, but there are concerns about the adverse events such as genital mycotic infections and urinary tract infections.[9] We aimed to pool the results of trials studying the efficacy and safety of canagliflozin in combination therapy for a duration of at least 26 weeks.
Objectives
To assess the efficacy and safety of canagliflozin in combination therapy among patients with inadequately controlled T2DM.
METHODS
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Type of participants
Patients with T2DM inadequately controlled with the use of glucose lowering agents, diet, and exercise.
Type of interventions
Treatment with canagliflozin (100 mg or 300 mg once daily) for at least 26 weeks in combination with earlier regimen of oral glucose-lowering agents. The following comparisons were evaluated:
Canagliflozin 300 mg/day versus placebo after 26 weeks
Canagliflozin 100 mg/day versus placebo after 26 weeks
Canagliflozin 300 mg/day versus sitagliptin 100 mg/day after 52 weeks.
Types of outcome measures
Primary outcomes
Effect on HbA1c levels after 26 weeks
Effect on fasting plasma glucose (FPG) levels after 26 weeks
Effect on body weight after 26 weeks.
Secondary outcomes
Effect on high density lipoprotein (HDL-C) levels after 26 weeks
Effect on triglyceride levels after 26 weeks
Effect on LDL-C levels after 26 weeks
Incidence of adverse events such as urinary tract infections and genital mycotic infections after 52 weeks
Effect on HbA1c, FPG levels and body weight after 52 weeks.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published and unpublished).
Electronic searches
Two independent (KK and NL) reviewers independently searched the following databases on 09 June 2015 using the search terms mentioned below with no limit of time of publication. Cochrane Central Register of Controlled Trials (CENTRAL) the Cochrane Library; MEDLINE; EMBASE, LILACS, and IndMed. We also searched the WHO clinical trial registry platform, ClinicalTrials.gov for ongoing trials. The search terms were “canagliflozin” and “T2DM.”
Searching other resources
We searched Conference Proceedings Citation Index for relevant material.[10] We contacted researchers/authors of the included studies for data input. We checked the reference lists of existing reviews and all trials identified by the above methods.
Data collection and analysis
Selection of studies
Two authors (KK and NL) independently screened the literature search results and obtained the full reports of all potentially relevant trials. KK and NL independently applied the inclusion criteria to the full reports using an eligibility form and scrutinized publications to ensure each trial was included only once. We resolved any disagreements through discussion with a third author.
Data extraction and management
KK and NL independently extracted data using specifically developed data extraction forms. We had access to the supplementary files of the included studies. We resolved any disagreements through discussion with all of the review authors. We contacted the corresponding publication author in the case of unclear information or missing data. For each outcome, we aimed to extract the number of participants randomized and the number analyzed in each treatment group. For dichotomous outcomes, we recorded the number of participants experiencing the event and the number assessed in each treatment group. For continuous outcomes, we used least mean squares and standard error and then calculated the standard deviation. We included only those clinical trials where the patients were already taking blood glucose-lowering oral agents.
Assessment of risk of bias in included studies
KK and NL independently assessed the risk of bias of each trial using the Cochrane risk of bias form.[11] We resolved any disagreements by discussion among review authors. Six components were assessed: Generation of the randomization sequence, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting and other biases (such as the trial stopped early). We categorized our judgments as either “low,” “high,” or “unclear” risk of bias, and described our reasons for doing so. We recorded our judgments and justifications in the risk of bias tables for each included study and generated a risk of bias summary graph and figure.
Measures of treatment effect
We calculated the results using risk ratios (RRs) for dichotomous data and mean difference values for continuous data, and presented these effect estimates with 95% confidence intervals (CIs). We had planned to calculate time-to-event outcomes as safety data, but due to lack of availability of results at 26 weeks, we did not use it for comparison between canagliflozin and placebo. It was used for comparison between canagliflozin and sitagliptin at 52 weeks.
Unit of analysis issues
For dichotomous outcomes, both the sample size and the number of people with events were added across the groups. For continuous outcomes, we combined means and standard deviations using methods described in the Cochrane Handbook for Systematic Reviews of Interventions.[12]
Dealing with missing data
The data were analyzed according to the intention-to-treat (ITT) principle (all randomized participants should be analyzed in the groups to which they were originally assigned). Relevant missing data were to be obtained from the respective authors, if feasible. Evaluation of important numerical data such as screened, eligible, and randomized patients as well as ITT and per-protocol (PP) population was carefully performed.
Assessment of heterogeneity
The statistical heterogeneity was analyzed by looking at the forest plots for overlapping CIs, applying the χ2 test (P < 0.10 considered statistically significant) and the I2 statistic (I2 value < 50% used to denote moderate levels of heterogeneity).
Data synthesis
We analyzed the data using review manager (RevMan) 5.3 of Cochrane collaboration. For the quantitative analysis, we used the fixed-effect meta-analysis.[12] Random effect model was used, when significant heterogeneity was present. We used the fixed effect model for calculating the change in HbA1c levels, FPG levels and body weight in canagliflozin 300 mg, and placebo arms after 26 weeks. The statistical analysis was performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions. Where heterogeneity was very high such that meta-analysis was not appropriate, we displayed the results in tables but did not combine the results.
RESULTS
Description of studies
Results of the search
We conducted the literature search up to 09 June 2015 and identified 124 references [Figure 1]. This initial search of randomized, controlled trials led to 22 results (from MEDLINE), 38 from Cochrane central, 24 from EMBASE, 38 from LILACS, and 2 from other sources. After excluding the duplicate reports, 55 reports were screened and then 26 were excluded. Then 29 studies were evaluated for eligibility. Five clinical studies were included for meta-analysis.
Figure 1.
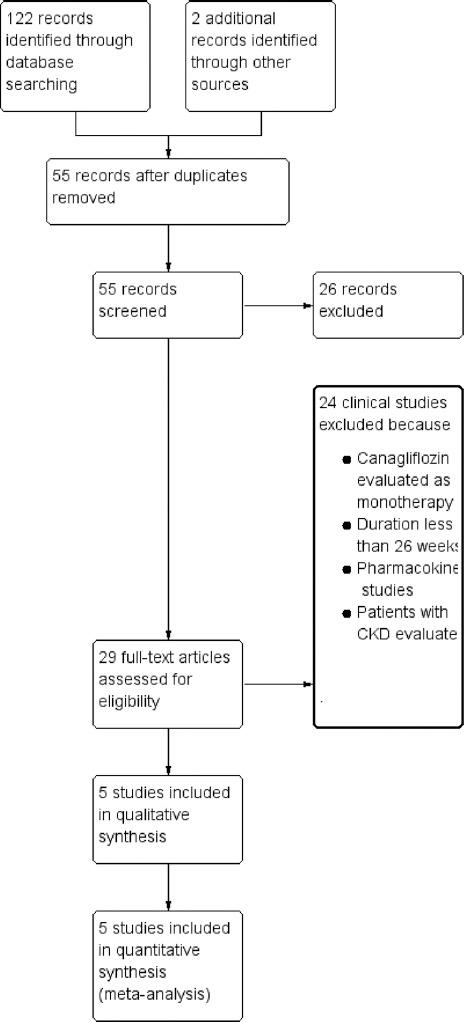
Study flow diagram
Included studies
Five clinical studies enrolling 3565 patients were included in the quantitative analysis [Table 1 - characteristics of included studies].
Table 1.
Characteristics of included studies

Design
These were randomized, double-blind clinical trials. All the trials had multinational design.
Intervention
All studies had compared canagliflozin with placebo. In three studies, there were three arms of the studies, that is, canagliflozin 100 mg and 300 mg once daily and placebo arms for the first 26 weeks in the first phase. In the following second phase (26–52 weeks), patients of placebo arm were administered sitagliptin 100 mg once a day and evaluated at the end of the study.[13,14,15] Lavalle-Gonzalez et al. had taken another arm of sitagliptin 100 mg once daily and Schernthaner et al. had only two arms of canagliflozin 300 mg once daily and sitagliptin 100 mg once a day.[16,17] All these studies had evaluated efficacy as primary (effect on HbA1c levels after 26 weeks) and secondary end points (FPG levels, body weight after 26 weeks, and HbA1c levels after 52 weeks) and monitored the safety and tolerability at 26 and 52 week interval.
Excluded studies
The reasons for exclusion of the studies are mentioned in table. Most of RCTs have evaluated the efficacy and tolerability of canagliflozin as monotherapy and some have assessed its efficacy among patients with chronic renal failure [Table 2].[18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]
Table 2.
Characteristics of excluded studies
Risk of bias in the included studies
Overall, the included studies suggested low risk of bias as these studies generally had a randomized controlled, double-blind design, typically employing an ITT analysis [Figure 2]. Inter-rater agreement for the key quality indicators such as randomization, concealment of allocation, and blinding was complete with no full publication necessary to be discussed by a third author. Figure 3 shows a summary of the judgments of the risk of bias for each domain in each of the included trials.
Figure 2.
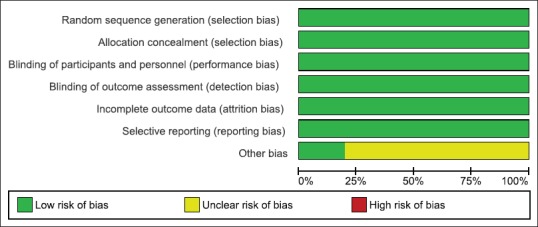
Risk of bias summary: Review authors’ judgments about each risk of bias item for each included study
Figure 3.

Risk of bias graph: Review authors’ judgments about each risk of bias item presented as percentages across all included studies
The included studies had allocation concealment thus avoiding the risk of bias. All studies employed a double-blind design. Most publications reported an ITT analysis using the last observation carried forward method to impute missing values. No publication indicated selective outcome reporting.
Effect of interventions
See summary of findings for the main comparison.
Canagliflozin 300 mg once a day versus placebo
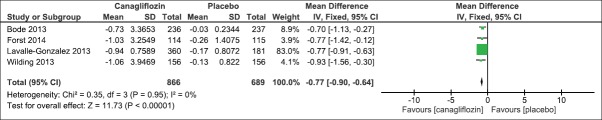
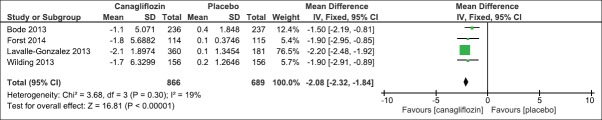
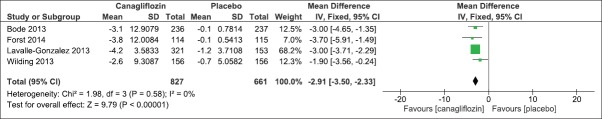
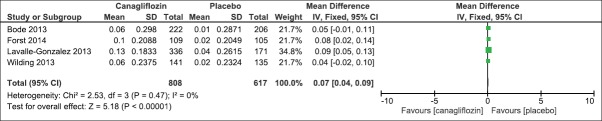
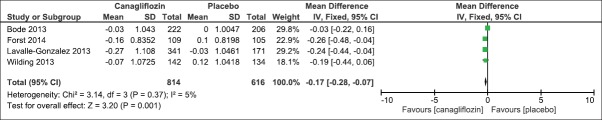
We evaluated the effect of canagliflozin 300 mg once daily as compared to placebo on HbA1c and FPG levels after 26 weeks. Four trials had reported the mean change as compared to baseline. There was a significant mean difference in HbA1c levels (IV Fixed − 0.77, 95% CI [−0.90, −0.64], P < 0.00001) [Analysis 1.1, Figure 4] and FPG levels (IV Fixed − 2.08; 95% CI [−2.32, −1.84], P < 0.00001) in the canagliflozin arm as compared to placebo [Analysis 1.2, Figure 5]. Similarly, there was a significant mean difference in effect on body weight [Analysis 1.3, Figure 6], systolic blood pressure [Analysis 1.4, Figure 7], HDL-C [Analysis 1.5, Figure 8], and triglyceride levels [Analysis 1.6, Figure 9] favoring canagliflozin 300 mg once a day as compared to placebo. The clinical studies had shown a numerical increase in LDL-C levels and this increase significantly favors placebo over canagliflozin [Analysis 1.7, Figure 10].
Figure 4.
Forest plot of comparison: 1 canagliflozin 300 mg once daily versus placebo after 26 weeks, outcome: 1.1 effect on hemoglobin A1c levels
Figure 5.
Forest plot of comparison: 1 canagliflozin 300 mg once daily versus placebo after 26 weeks, outcome: 1.2 effect on fasting plasma glucose levels
Figure 6.
Forest plot of comparison: 1 canagliflozin 300 mg once daily versus placebo after 26 weeks, outcome: 1.3 effect on body weight
Figure 7.
Forest plot of comparison: 1 canagliflozin 300 mg once daily versus placebo after 26 weeks, outcome: 1.4 effect on systolic blood pressure
Figure 8.
Forest plot of comparison: 1 canagliflozin 300 mg once daily versus placebo after 26 weeks, outcome: 1.5 effect on high-density lipoprotein-C levels
Figure 9.
Forest plot of comparison: 1 canagliflozin 300 mg once daily versus placebo after 26 weeks, outcome: 1.6 effect on triglyceride levels
Figure 10.
Forest plot of comparison: 1 canagliflozin 300 mg once daily versus placebo after 26 weeks, outcome: 1.7 effect on low-density lipoprotein-C levels
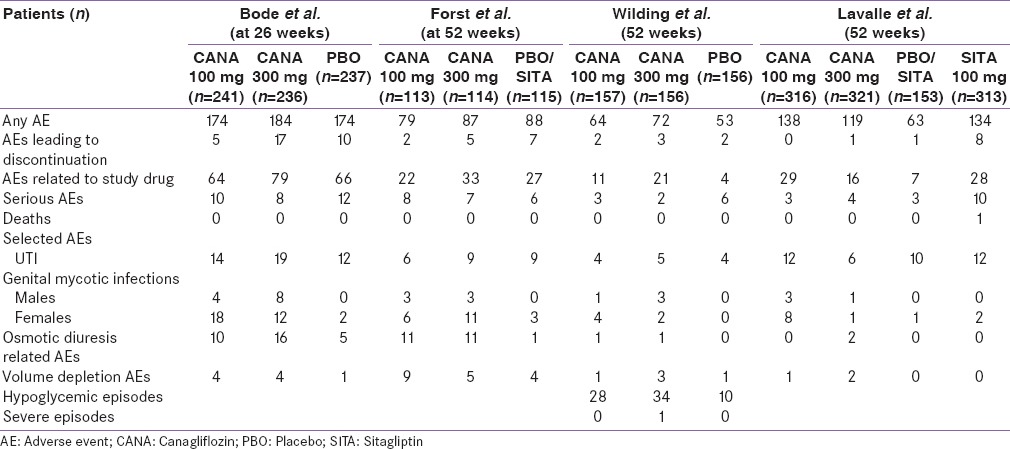
The incidence of adverse effects in the two arms could not be compared quantitatively as the clinical studies except Bode et al., have not described this information at 26 weeks. The number of adverse effects has been listed in Table 3 for comparison.
Table 3.
Adverse events observed in the included studies
Canagliflozin 100 mg once a day versus placebo
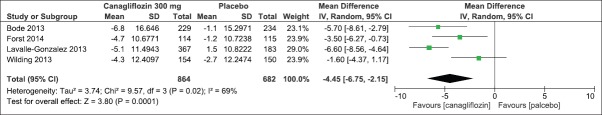
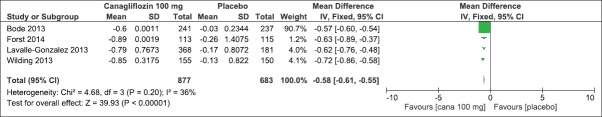
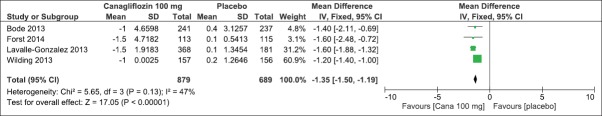
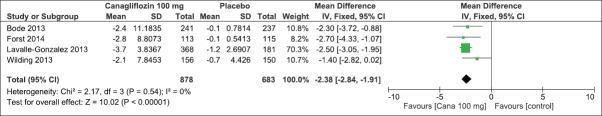
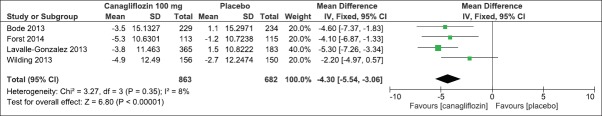
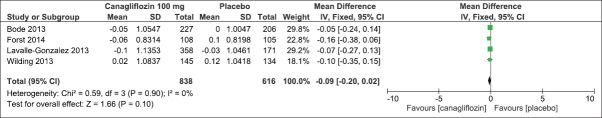
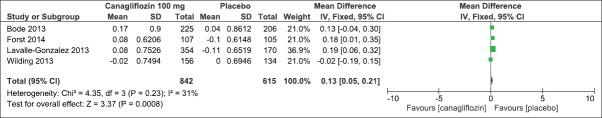
On evaluation of the above-mentioned four clinical studies, there was a significant mean difference in decrease in HbA1c (IV, Fixed − 0.58 [−0.61, −0.55], P < 0.0001) [Analysis 2.1, Figure 11] and FPG levels [IV, Fixed −1.35 95% CI [−1.50, −1.19], P < 0.0001) [Analysis 2.2, Figure 12] in favor of canagliflozin. The mean difference was also significant for effect on body weight [Analysis 2.3, Figure 13], systolic blood pressure [Analysis 2.4, Figure 14], and HDL-C levels [Analysis 2.5, Figure 15] and nonsignificant for triglyceride levels [Analysis 2.6, Figure 16] in favor of canagliflozin 100 mg as compared to placebo. Similar to canagliflozin 300 mg dose, even 100 mg once a day also led to increase in LDL-C levels and the comparison was in favor of placebo [Analysis 2.7, Figure 17]. As mentioned earlier, the incidence of adverse events could not be compared at 26 weeks.
Figure 11.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.1 effect on hemoglobin A1c levels
Figure 12.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.2 effect on fasting plasma glucose levels
Figure 13.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.3 effect on body weight
Figure 14.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.4 effect on systolic blood pressure
Figure 15.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.5 effect on high-density lipoprotein-C levels
Figure 16.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.6 effect on triglyceride levels
Figure 17.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.7 effect on low-density lipoprotein-C levels
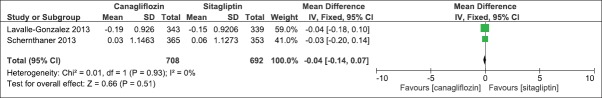
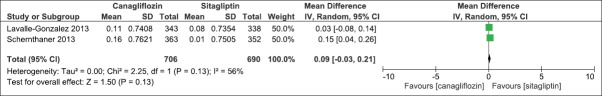
Canagliflozin 300 mg once daily versus sitagliptin 100 mg once daily
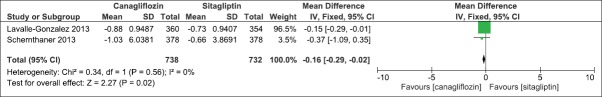
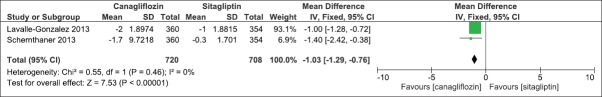
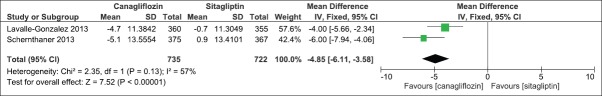
We compared the effect of canagliflozin 300 mg once daily and sitagliptin 100 mg once daily when added to an earlier regime of anti-hyperglycemic agents in two clinical studies.[16,17] The duration of the clinical studies was 52 weeks. There was a significant mean difference in the effect on HbA1c levels (IV, Fixed − 0.16, 95% CI [−0.29,-0.02], P = 0.02) [Analysis 3.1, Figure 18]. There was also a significant mean difference in FPG levels (IV, Fixed − 1.03, 95% CI [−1.29, −0.76], P < 0.0001) [Analysis 3.2, Figure 19] and body weight (IV, Fixed − 2.49, 95% CI [−3.00, −1.97], P < 0.0001) [Analysis 3.3, Figure 20]. There was a significant difference in decrease in systolic BP [Analysis 3.4, Figure 21] and increase in HDL-C levels [Analysis 3.5, Figure 22] in favor of canagliflozin, but a nonsignificant difference in decrease in triglyceride levels [Analysis 3.6, Figure 23]. Both canagliflozin and sitagliptin groups had witnessed a numerical increase in LDL-C levels in the clinical studies, but the comparative mean difference in favor of sitagliptin was not significant [Analysis 3.7, Figure 24].
Figure 18.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.1 effect on hemoglobin A1c levels
Figure 19.
Forest plot of comparison: 2 canagliflozin 100 mg once daily versus placebo after 26 weeks, outcome: 2.6 effect on triglyceride levels
Figure 20.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.3 effect on body weight
Figure 21.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.4 effect on systolic blood pressure
Figure 22.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.5 effect on high-density lipoprotein-C levels
Figure 23.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.6 effect on triglyceride levels
Figure 24.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.7 effect on low-density lipoprotein-C levels
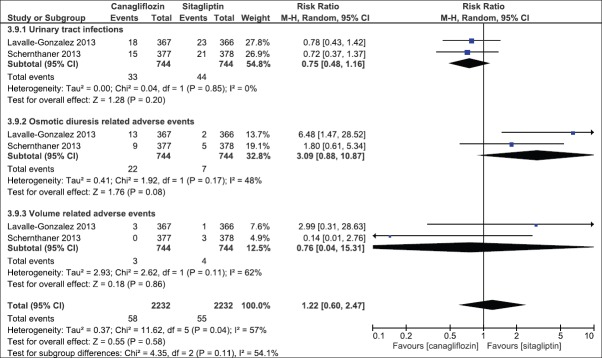
Incidence of adverse effects
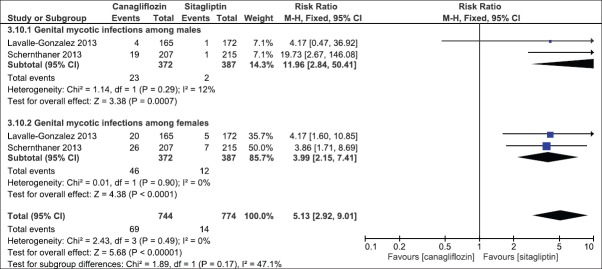
There was a nonsignificant difference between the two groups with respect to occurrence of adverse effects (RR: 0.98, 95% CI: 0.92, 1.05, P = 0.57) [Analysis 3.8, Figure 25]. In addition, the occurrence of osmotic diuretic-related adverse effects and volume depletion-related adverse events was not significantly different between the two groups [Analysis 3.9, Figure 26]. The risk of development of genital mycotic infections was higher with canagliflozin among both males (MH, Fixed 11.96, 95% CI [2.84–50.41], P = 0.0007) and females (MH, Fixed 3.99, 95% CI [2.15–7.40], P < 0.0001) [Analysis 3.10, Figure 27] as compared to sitagliptin.
Figure 25.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.8 adverse events
Figure 26.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.9 selected adverse events
Figure 27.
Forest plot of comparison: 3 canagliflozin 300 once daily versus sitagliptin 100 mg after 52 weeks, outcome: 3.10 genital mycotic infections
DISCUSSION
Summary of main results
Five good quality randomized studies comparing two different doses of canagliflozin with placebo or sitagliptin among patients inadequately controlled with oral anti-hyperglycemic agents over 26 weeks were included for quantitative analysis.[13,14,15,16,17] The results show that both the doses of canagliflozin (100 mg/day and as 300 mg/day) when used in combination with other oral anti-hyperglycemic agents led to a significant decrease in HbA1c levels, FPG levels, and body weight as compared to placebo over a period of 26 weeks. In addition, canagliflozin significantly decreased the systolic blood pressure, mean triglyceride levels, and increased HDL-C levels as compared to placebo. No quantitative analysis was done for the incidence of adverse effects at 26 weeks because of the unavailability of the results.[14,15,16] In all the included studies, there was a numerical increase in LDL-C levels, which was nonsignificant in favor of placebo and sitagliptin upon analysis. Comparison of canagliflozin 300 mg once daily and sitagliptin 100 mg once daily shows a significant difference in the decrease of HbA1c levels, FPG levels, and body weight in favor of canagliflozin at 52 weeks.[16,17] The incidence of total adverse effects and selected adverse effects such as urinary tract infections, osmotic dieresis related, and volume-related adverse effects were nonsignificantly different between the two groups. However, the incidence of genital mycotic infections among both males and females was significantly more in the canagliflozin arm as compared to sitagliptin. Although the included clinical studies, that is, Bode et al., Forst et al., and Wilding et al. had reported the efficacy and safety results at 52 weeks as well, we did not include these results, as the patients of the placebo arm had been shifted to a comparator group at 26 weeks and then followed up for next 26 weeks.[13,14,15,16] Thus, the comparator drug administered for the later 26 weeks could not be compared with canagliflozin given for 52 weeks. With reference to the comparison of canagliflozin and sitagliptin, there is a need to have more long-term studies for conclusive results.
The need of new glucose-lowering agents is based on multiple factors. First, T2DM is a chronic disease that often requires combination therapy with glucose-lowering agents as the disease progresses. Second, improved glycemic control is associated with significantly decreased rates of microvascular and neuropathic complications.[2,42] Intensive early management of T2DM patients may also reduce the long-term cardiovascular disease rates (both fatal and nonfatal myocardial infarction and sudden death).[2] Third, many of the available glucose-lowering agents have adverse effects such as weight gain and increased risk of hypoglycemia. Finally, it will be advantageous if the glucose-lowering agents can simultaneously have beneficial effects on body weight, blood pressure, and lipid profile, that is, triglyceride, LDL-C, and HDL-C levels. Another reason is that more patients with T1DM and T2DM are living into older age and there is lack of clinical evidence for their management.[2] Hence, it is vital to have efficacious and safe drugs for the management of T2DM.
Canagliflozin and other SGLT2 inhibitors, the latest drugs to be added to the pool of glucose-lowering agents, have the advantage of an insulin-independent mechanism of action, while on the downside; these drugs have been associated with some adverse effects. US FDA has given a warning regarding the risk of increased incidence of keto-acidosis with canagliflozin.[43] This safety issue is being investigated by the drug authorities and in future, a modification in drug prescribing may be required. Furthermore, there is a need to evaluate the incidence of genital mycotic infections and the increase in LDL-C levels. Long-term randomized and observational safety studies comparing canagliflozin with available glucose-lowering agents can help in assessing the incidence of adverse effects.
As we included clinical studies evaluating canagliflozin in combination therapy, we could not analyze the status of canagliflozin in monotherapy. Quantitative analysis of canagliflozin monotherapy has also shown that canagliflozin is significantly more efficacious than placebo in decreasing HbA1c, FPG, and body weight.[44,45] Canagliflozin has also been shown to be efficacious and safe among patients with T2DM and chronic kidney disease. There was a significant decrease in HbA1c levels, body weight, and blood pressure over 52 weeks.[41]
Strengths and weaknesses of the review
One of the important strengths of the review includes the assessment of efficacy and safety of canagliflozin in combination therapy in two different doses with placebo and a comparator, sitagliptin. We have searched the databases till June 2015, thus trying to include all the possible data related to canagliflozin. This review consists of published data only. Upon completion of a thorough search of all major databases with no language restrictions, we believe that all relevant studies were identified. Two review authors assessed the trials for inclusion in the review and the risk of bias, and a third review author adjudicated whether there was any discrepancy. Most of the reviews have evaluated canagliflozin in monotherapy and for shorter duration of administration, that is, 12–18 weeks.
One weakness of the review includes the nonavailability of safety data from the included studies at 26 weeks for quantitative analysis. Information about the incidence of adverse effects in comparison to placebo at 26 weeks was available from Bode et al., only.[13] Rest of the studies had mentioned the safety profile at 52 weeks, which could not be compared as in these studies the initial placebo arm had been shifted to a comparator group at 26 weeks.[14,15,16] Hence, this arm could not be compared with canagliflozin arms.
CONCLUSION
Canagliflozin (100 mg and 300 mg once daily) significantly decreases HbA1c levels, FPG levels, body weight, systolic blood pressure, and triglyceride levels, and simultaneously also increases HDL-C levels when used in combination therapy among patients with inadequate glycemic control as compared to placebo. In view of increasing concern about the safety profile of canagliflozin, there is a need of long-term studies of canagliflozin as compared to the available glucose-lowering agents.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Global Diabetes Atlas. [Last accessed on 2015 Sep 29]. Available from: https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf .
- 2.American Diabetes Association. Standards of medical care in diabetes-2015: Summary of revisions. Diabetes Care. 2015;38(Suppl:S4) doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 3.Powers A, D’Alessio D. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycemia. In: Brunton LL, D’Alessio D, editors. Goodman and Gilman's the pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill; 2011. pp. 1255–65. [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Invokana Prescribing Information. [Last accessed on 2015 Sep 29]. Available from: http://www.invokanahcp.com/prescribing-information.pdf .
- 6.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 7.Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–7. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 8.Whalen K, Miller S, Onge ES. The role of sodium-glucose co-transporter 2 inhibitors in the treatment of type 2 diabetes. Clin Ther. 2015;37:1150–66. doi: 10.1016/j.clinthera.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Riser Taylor S, Harris KB. The clinical efficacy and safety of sodium glucose cotransporter-2 inhibitors in adults with type 2 diabetes mellitus. Pharmacotherapy. 2013;33:984–99. doi: 10.1002/phar.1303. [DOI] [PubMed] [Google Scholar]
- 10.Conference Proceedings Citation Index. [Last accessed on 2015 Jun 22]. Available from: http://www.thomsonreuters.com/en/search-results.html?q=invokana and sp_cs=UTF-8 and sp_k=English-All .
- 11.Higgins JP, Altman DG, Sterne JA. Higgins JP, Green S, editors. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). Ch. 8. The Cochrane Collaboration. 2011. [Last accessed on 2015 Sep 29]. Available from: http://www.cochrane-handbook.org .
- 12.Deeks JJ, Higgins JP, Altman DG. Higgins JP, Green S, editors. Analyzing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). Ch. 9. The Cochrane Collaboration. 2011. [Last accessed on 2015 Sep 29]. Available from: http://www.cochrane-handbook.org .
- 13.Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp Pract (1995) 2013;41:72–84. doi: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 14.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–77. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: A randomised trial. Int J Clin Pract. 2013;67:1267–82. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia. 2013;56:2582–92. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: A 52-week randomized trial. Diabetes Care. 2013;36:2508–15. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–50. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 19.Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–45. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 20.Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601–10. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Galvez G, Kim KA, Jodar E, Alba M, Tong C, Meininger G. Effect of canagliflozin (CANA) in patients with type 2 diabetes mellitus (T2DM) who were, or were not, on antihyperglycemic agents (AHAS) at screening. Diabetes. 2014;63:A280. [Google Scholar]
- 22.Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15:1136–45. doi: 10.1111/dom.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: A 24-week, randomized, double-blind, placebo-controlled, phase III study. Expert Opin Pharmacother. 2014;15:1501–15. doi: 10.1517/14656566.2014.935764. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki N, Kondo K, Yoshinari T, Ishii M, Sakai M, Kuki H, et al. Pharmacokinetic and pharmacodynamic profiles of canagliflozin in Japanese patients with type 2 diabetes mellitus and moderate renal impairment. Clin Drug Investig. 2014;34:731–42. doi: 10.1007/s40261-014-0226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langslet G, Davidson JA, Valentine V, Vijapurkar U, Canovatchel W, Meininger G. Canagliflozin reduces both HbA1c and body weight in patients with type 2 diabetes mellitus on background metformin. Diabetologia. 2014;57:S348. [Google Scholar]
- 26.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS) – A randomized placebo-controlled trial. Am Heart J. 2013;166:217–223.e11. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Nicolle LE, Capuano G, Ways K, Usiskin K. Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin. 2012;28:1167–71. doi: 10.1185/03007995.2012.689956. [DOI] [PubMed] [Google Scholar]
- 28.Nicolle LE, Capuano G, Fung A, Usiskin K. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad Med. 2014;126:7–17. doi: 10.3810/pgm.2014.01.2720. [DOI] [PubMed] [Google Scholar]
- 29.Nyirjesy P, Zhao Y, Ways K, Usiskin K. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin. 2012;28:1173–8. doi: 10.1185/03007995.2012.697053. [DOI] [PubMed] [Google Scholar]
- 30.Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: A pooled analysis of clinical studies. Curr Med Res Opin. 2014;30:1109–19. doi: 10.1185/03007995.2014.890925. [DOI] [PubMed] [Google Scholar]
- 31.Polidori D, Sha S, Ghosh A, Plum-Mörschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:E867–71. doi: 10.1210/jc.2012-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu R, Capuano G, Meininger G. Efficacy and safety of twice-daily treatment with canagliflozin, a sodium glucose co-transporter 2 inhibitor, added on to metformin monotherapy in patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2014;1:54–60. doi: 10.1016/j.jcte.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–8. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–72. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 35.Sinclair A, Bode B, Harris S, Vijapurkar U, Mayer C, Fung A, et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: A pooled analysis of clinical studies. BMC Endocr Disord. 2014;14:37. doi: 10.1186/1472-6823-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–82. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenlöf K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: Findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163–75. doi: 10.1185/03007995.2013.850066. [DOI] [PubMed] [Google Scholar]
- 38.Stein P, Berg JK, Morrow L, Polidori D, Artis E, Rusch S, et al. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces post-meal glucose excursion in patients with type 2 diabetes by a non-renal mechanism: Results of a randomized trial. Metabolism. 2014;63:1296–303. doi: 10.1016/j.metabol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Traina S, Guthrie R, Slee A. The impact of weight loss on weight-related quality of life and health satisfaction: Results from a trial comparing canagliflozin with sitagliptin in triple therapy among people with type 2 diabetes. Postgrad Med. 2014;126:7–15. doi: 10.3810/pgm.2014.05.2752. [DOI] [PubMed] [Google Scholar]
- 40.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–27. doi: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 42.UK Prospective Diabetes Study. [Last accessed on 2015 Jul 10]. Available from: https://www.dtu.ox.ac.uk/UKPDS/trialresults.php .
- 43.US FDA. [Last accessed on 2015 Jul 03]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm .
- 44.Inagaki N, Kondo K, Yoshinari T, Kuki H. Efficacy and safety of canagliflozin alone or as add-on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: A 52-week open-label study. J Diabetes Investig. 2015;6:210–8. doi: 10.1111/jdi.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usiskin K, Kline I, Fung A, Mayer C, Meininger G. Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: Pooled analysis of phase 3 study results. Postgrad Med. 2014;126:16–34. doi: 10.3810/pgm.2014.05.2753. [DOI] [PubMed] [Google Scholar]