Abstract
Background
A paucity of therapeutic options is available to treat men with metastatic castration-resistant prostate cancer (mCRPC). However, recent developments in our understanding of the disease have resulted in several new therapies that show promise in improving overall survival rates in this patient population.
Methods
Agents approved for use in the United States and those undergoing clinical trials for the treatment of mCRPC are reviewed. Recent contributions to the understanding of prostate biology and bone metastasis are discussed as well as how the underlying mechanisms may represent opportunities for therapeutic intervention. New challenges to delivering effective mCRPC treatment will also be examined.
Results
New and emerging treatments that target androgen synthesis and utilization or the microenvironment may improve overall survival rates for men diagnosed with mCRPC. Determining how factors derived from the primary tumor can promote the development of premetastatic niches and how prostate cancer cells parasitize niches in the bone microenvironment, thus remaining dormant and protected from systemic therapy, could yield new therapies to treat mCRPC. Challenges such as intratumoral heterogeneity and patient selection can potentially be circumvented via computational biology approaches.
Conclusions
The emergence of novel treatments for mCRPC, combined with improved patient stratification and optimized therapy sequencing, suggests that significant gains may be made in terms of overall survival rates for men diagnosed with this form of cancer.
Introduction
Prostate cancer is the second most common cancer in American men with approximately 233,000 newly diagnosed cases in 2014.1 With an aging population, the incidence of prostate cancer is likely to continue to increase. Patients whose disease is detected at an early stage benefit from a range of treatment strategies, including radiotherapy and prostatectomy, with survival rates near 100%.2 However, the clinical reality is that many men present with advanced stages of the disease. Currently, the main treatment option for men with advanced cancer is hormone therapy. Historic contributions from Huggins and Hodges3 in 1941 revealed that removing androgens could inhibit the progression of prostate cancer. These early observations paved the way for the development of androgen-deprivation therapy — either surgically or chemically — which has remained the standard treatment for men with advanced disease for the last 70 years. Despite the initial response to androgen deprivation for most men, the disease typically progresses to a castration-resistant state within 18 to 24 months.4
Castration-resistant prostate cancer (CRPC) is defined by disease progression that, despite chemical castration, is often indicated by rising levels of prostate-specific antigen (PSA).5 The development of resistance to hormonal intervention and why the disease progresses is not fully understood, although some mechanisms have been demonstrated, with the majority focusing on the continued androgen receptor (AR) activity in addition to TMPRSS2/ERG fusion, PTEN, Nkx3.1, and EGR1. As the disease progresses, the CRPC ultimately metastasizes (mCRPC). Patients with mCRPC have a poor prognosis and a predicted survival rate of fewer than 2 years from the initial time of progression, comprising a large portion of the 30,000 prostate cancer-related deaths per year.6,7 Currently, mCRPC is an incurable disease and represents a major clinical hurdle.
Prostate cancer preferentially metastasizes to bone.8 As the disease transitions from castration sensitive to castration resistant, the incidence of bone metastasis increases, with more than 90% of patients with mCRPC developing bone metastases.9,10 Patients with mCRPC who are symptomatic are at a high risk for skeletal-related events (SREs), including spontaneous fracture and spinal cord compression, that are a source of significant pain and decreased quality of life.11 Pain from the metastases is a major component of the disease and is an important aspect to be considered regarding a patient’s treatment regimen. Depending on the level of pain, medications ranging from ibuprofen to morphine are prescribed.12 Because prostate to bone metastases are primarily bone-forming sclerotic lesions, bone scanning using technetium-99m is often preferred for diagnosis due to the incorporation of the radionuclide tracer into regions of new bone formation by osteoblasts.13 Magnetic resonance imaging (MRI) and positron emission tomography (PET)/computed tomography (CT) are also used for detection. A trial comparing 18F–sodium fluoride PET/CT, 18F-fluorodeoxyglucose PET/CT, MRI, and technetium-99m identified strengths for each modality.14 However, the ability to detect occult or micrometastases less than 5 mm remains a current limitation for each imaging technique.
Approved Therapeutic Options
Currently, mCRPC remains incurable, and many treatment options are palliative in nature. However, the treatment landscape of mCRPC is expanding both in broad-spectrum and targeted therapies that are likely to positively impact overall survival rates within the next decade. This expansion began with docetaxel, which, in 2004, was the first therapy to provide improved survival rates to patients with mCRPC. However, many patients develop resistance.15 To combat this issue, 5 new agents have received approval by the US Food and Drug Administration (FDA) to treat mCRPC since 2010 (abiraterone acetate, enzalutamide, cabazitaxel, radium-223, and sipuleucel-T).16 Some of these agents may be administered in combination with steroids, such as prednisone, which has been shown to decrease testosterone levels and reduce tumor growth as well as counteract adverse events (eg, nausea, allergic reactions, inflammation, pain).17,18 Recently FDA-approved agents that target the cancer and host compartments are discussed below and are also illustrated in Fig 1.
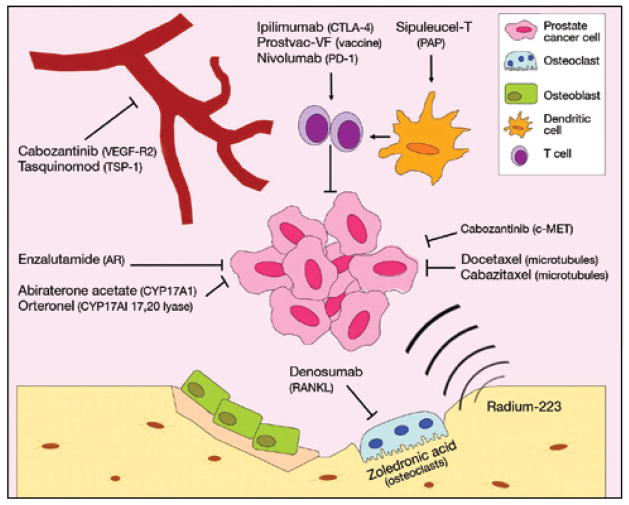
Fig 1.
Approved and developing mCRPC therapies and their targets. mCRPC has experienced a rapid expansion of treatment options over the last decade. Better understanding of mechanisms of progression has allowed for the improvement of broad-acting options such as chemotherapy and hormonal therapy as well as the development of novel targeted therapies to modulate the immune system and microenvironment. mCRPC = metastatic castration-resistant prostate cancer.
Targeting Metastatic Castration-Resistant Prostate Cancer Cells
One of the defining measures of mCRPC is resistance to androgen deprivation. The mechanism of castration resistance is not fully understood but inroads have been made. For example, prostate cancer cells circumvent castration by overexpressing and increasing the sensitivity of the AR to residual androgens, acquiring AR gene mutations that lead to functional gain or promiscuous ligand interactions, splice variants resulting in constitutive AR activation, and post-translational modifications affecting the stability, localization, and activity of the receptor.19 Alternative methods utilized by prostate cancer cells to synthesize dihydrotestosterone (DHT) have also been shown to circumvent androgen deprivation methods.20–22 Efforts to target DHT synthesis have resulted in FDA-approved androgen deprivation therapy (ADT) options. Abiraterone acetate is one such option that works by inhibiting the activity of the CYP17A1 enzyme, thereby preventing androgen synthesis. Abiraterone has improved the overall survival and radiographic progression-free survival rates of men with mCRPC.23,24 Another therapeutic strategy for preventing androgen utilization by mCRPC cells is to directly target the AR with reagents such as flutamide, nilutamide, and bicalutamide. Enzalutamide was recently approved for the treatment of mCRPC in a postdocetaxel setting without the administration of corticosteroids.25,26 Enzalutamide has a superior affinity to the AR compared with other AR antagonists and works by preventing nuclear translocation of the receptor, DNA binding, and recruitment of coactivators of the AR to increase overall survival rates and delay the onset of SREs.27–29 Results of a phase 3 trial demonstrated enzalutamide activity in patients naive to chemotherapy, and FDA approval of enzalutamide as a first-line therapeutic option for mCRPC may be on the horizon.30
A list of approved therapies for the treatment of mCRPC appears in Table 1.15,23,24,27,28,31–36
Table 1.
Approved Therapies for the Treatment of Metastatic Castration-Resistant Prostate Cancer
| Drug | Target | Effect |
|---|---|---|
| Abiraterone acetate | CYP17A1 | Reduces circulating testosterone levels23,24 |
| Cabazitaxel | Microtubules | Microtubule stabilization, interrupts cell cycle31 |
| Denosumab | RANKL | Decreases bone resorption34 |
| Docetaxel | Microtubules | Microtubule stabilization, interrupts cell cycle15,36 |
| Enzalutamide | AR | AR antagonism, prevents signaling27,28 |
| Radium-223 | Bone | Localized radiation35 |
| Sipuleucel-T | Ex vivo activation of PBMCs via GM-CSF and PAP | T-cell activation32 |
| Zoledronic acid | Osteoclasts | Decreases bone resorption33 |
AR = androgen receptor, GM-CSF = granulocyte-macrophage colony-stimulating factor, PAP = prostatic acid phosphatase, PBMC = peripheral blood mononucleated cell, RANKL = receptor activator of nuclear κB ligand.
In addition to ADT strategies, taxane-derived chemotherapies are commonly used to treat mCRPC. Docetaxel was the first therapy to demonstrate a beneficial effect on overall survival rates accompanied by improved quality of life for men with mCRPC, and it has since become the standard therapy for mCRPC.15,36 Cabazitaxel is a more recent derivative of the taxoids that has shown increases in overall survival rates, improvements in progression-free survival rates, and improved PSA response rates in men with mCRPC.31,37 Cabazitaxel-associated toxicities were minor, leading to the FDA approval of the therapy for the treatment of patients with mCRPC after treatment with docetaxel.38
Targeting the Microenvironment
Given the heterogeneity of mCRPCs and the likelihood of ADT/chemotherapy resistance, targeting the genetically stable host microenvironment supporting the mCRPC represents an attractive treatment approach. Immune evasion is a hallmark of cancer progression, and the goal of sipuleucel-T is to make mCRPC more visible to cytotoxic T cells.32,39 Sipuleucel-T is an autologous immunotherapy approved for the treatment of asymptomatic or minimally symptomatic mCRPC.40 Sipuleucel-T harnesses the properties of the patient’s immune system by collecting peripheral blood mono-nuclear cells and activating them ex vivo by exposing them to a fusion protein consisting of prostatic acid phosphatase (PAP; commonly expressed by prostate cancer cells) and granulocyte-macrophage colony-stimulating factor. Patients receive 3 separate infusions of the activated cells at 2-week intervals to generate PAP-expressing dendritic cells that activate T cells to recognize and eliminate PAP-expressing prostate cancer cells.32
Most mCRPCs arise in the bone matrix where they induce extensive bone remodeling by stimulating osteoblasts and osteoclasts. The process promotes the growth of the mCRPCs via the solubilization of bone matrix–sequestered growth factors, causing pain and SREs (eg, pathological fractures). Therefore, preventing the interaction of cancer and bone has been a major focus of treatment for several decades. Bisphosphonates, such as zoledronic acid, are reagents that can “stick” to bones undergoing remodeling; upon resorption by osteoclasts, they can induce apoptosis and limit the amount of cancer-induced bone disease.41 In the clinical setting, zoledronic acid has demonstrated a benefit for patients with mCRPC by delaying the time to SRE incidence.33 However, no increase in overall survival rates has been demonstrated. Receptor activator of nuclear κB ligand (RANKL) is a molecule critical for the maturation and activation of bone-resorbing osteoclasts. Denosumab is a fully humanized monoclonal antibody that prevents RANKL interaction with the RANK receptor.42 For patients with bone mCRPC, a significant delay has been demonstrated in the time to first SRE compared with zoledronic acid.34 Evidence suggests that denosumab may have direct effects on tumor burden, particularly tumor cells expressing RANK.43,44 Furthermore, preclinical in vivo animal studies have highlighted the efficacy of docetaxel/denosumab treatment in increasing median survival rates, suggesting that combination approaches with denosumab could enhance the overall survival rates of men with mCRPC.45
At the time of publication, the most recent agent to receive FDA approval for mCRPC is radium-223.46 The bone-seeking properties of radium-223 (and other similar radiopharmaceuticals) make it useful for the treatment of bone metastases. Although most radiopharmaceuticals emit β particles, radium-223 emits α particles to deliver more localized radiation (< 100 μm distance) to induce cell death via DNA damage.47 In a study of men with mCRPC previously treated with radiotherapy, radium-223 showed improved rates of overall survival, time to PSA progression, and reduced alkaline phosphatase levels (a measure of bone remodeling).35 In addition, radium-223 delays the time to first SRE.35 Previous radiopharmaceuticals used to treat mCRPC were effective at reducing pain alone. Therefore, radium-223 represents an important step forward for the field.46
Emerging Therapeutic Options
Despite the growing number of FDA-approved agents to treat mCRPC, room remains to improve upon the therapeutic options available to patients and clinicians. For example, although approximately 50% of patients with mCRPC will respond to docetaxel, most patients develop resistance and disease progression within 1 year of beginning treatment.36 However, some treatments that target cancer and support the microenvironment are currently in clinical trials that have the potential to provide health care professionals with new therapeutic options to treat men diagnosed with mCRPC (see Fig 1). A list of these experimental therapies appears in Table 2.32,48–57
Table 2.
Experimental Therapies for the Treatment of Metastatic Castration-Resistant Prostate Cancer
| Drug | Target | Effect | Study Results |
|---|---|---|---|
| Cabozantinib | c-MET VEGF-R2 |
Inhibits tyrosine kinase activity | Partial resolution of bone lesions, decreases number of CTCs, decreases pain51 |
| Custirsen | Clusterin | Improves response to docetaxel | Extended median survival, extends PFS, improves PSA declines52 |
| Ipilimumab | CTLA-4 | T-cell activation | Ongoing54,55 |
| Nivolumab | PD-1 | T-cell activation | Ongoing53 |
| Orteronel | CYP17A1 (17,20 lyase activity) | Reduces circulating testosterone levels | Decreases number of CTCs, improves radiographic PFS48,49 |
| Prostvac-VF | Delivery of PSA transgene | T-cell activation | Improves median survival32,56 |
| Tasquinimod | Thrombospondin S100A9 | Antiangiogenic, reduces MDSC recruitment | Improves median PFS, stable bone alkaline phosphatase levels50,57 |
CTC = circulating tumor cell, CTLA = cytotoxic T-lymphocyte antigen 4, MDSC = myeloid-derived suppressor cell, PD-1 = programmed cell death 1, PFS = progression-free survival, PSA = prostate-specific antigen, VEGF-R2 = vascular endothelial growth factor receptor 2.
Orteronel
Similar to abiraterone acetate, orteronel inhibits CYP17A1 to reduce circulating levels of testosterone. However, orteronel possesses specificity toward lyase activity, leaving the synthesis of adrenal cortisol unaltered.17,20 Therefore, orteronel is less likely than abiraterone acetate to require the concomitant administration of corticosteroids.25,58 Phase 2 trials demonstrated a significant reduction in serum levels of PSA that led to 10 partial responses and 22 cases of stable disease in 51 patients.59 Decreases in circulating tumor cells were also observed, thus serving as a further indication of efficacy. Based on these positive data, phase 3 trials were initiated; however, the results of one of those phase 3 trials indicated that orteronel administered in combination with prednisone failed to significantly impact overall survival rates compared with placebo but did provide a benefit in radiographic progression free survival rates in both chemotherapy naive and postchemotherapy mCRPC.48,49
Targeting the Microenvironment
Tasquinimod
In addition to the approval of some small molecule inhibitors, several novel inhibitors are, at the time of publication, in various phases of clinical trials for mCRPC. Tasquinimod, a quinoline-3-carboxamide derivative, is being investigated in men with mCRPC (NCT01234311, NCT00560482). Tasquinimod provides an antiangiogenic effect by upregulating thrombospondin-1 (TSP-1) and downregulating the gene expression of vascular endothelial growth factor (VEGF), the C-X-C chemokine receptor (CXCR) 4, and lysyl oxidase.60 It has also been shown to reduce the expression levels of C-X-C chemokine motif (CXCL) 12 and inhibit S100A9, both of which are important molecules implicated in tumorigenesis and angiogenesis.50,60–63 The results of a phase 2 trial in patients naive to chemotherapy showed improved rates of median progression-free survival (7.6 months vs 3.3 months).57 In addition, the study showed bone alkaline phosphatase levels, a correlate of bone turnover, were stabilized in patients receiving tasquinimod. Following the favorable outcome of the phase 2 trial, a phase 3 trial comparing tasquinimod to placebo was initiated in patients with mCRPC naive to chemotherapy.50
Cabozantinib
Cabozantinib is a tyrosine kinase inhibitor that blocks c-MET and VEGF receptor 2 and is already approved for the treatment of medullary thyroid cancer. This fact, combined with its oral administration, makes it a favorable candidate for further investigation and development in mCRPC. Phase 2 clinical trials have shown that cabozantinib results in partial resolution of bone lesions in 56% of patients and provided complete resolution in 19%.51 A total of 64% had an improvement in pain and 46% were able to decrease or discontinue narcotics.51 An additional exploratory analysis updated the results of this phase 2 trial and indicated a reduction of more than 30% in the bone scan lesion area and also indicated a reduction in circulating tumor cells.64 Multiple phase 3 trials focused on the treatment of mCRPC with cabozantinib are either ongoing or in the recruiting stages (NCT01428219, NCT01703065, NCT01995058, NCT01605227, NCT01834651, NCT01599793, NCT01522443, NCT01683994). At the time of publication, NCT01605227 failed to reach efficacy in men with mCRPC.
Custirsen
Custirsen is an antisense oligonucleotide that targets clusterin, a chaperone induced by stress and detected at elevated levels in several tumor types, including prostate cancer.65 Studies of clusterin have demonstrated its antiapoptotic and prosurvival activities in prostate cancer that are believed to be associated with docetaxel resistance.66 As such, inhibiting clusterin concomitantly with docetaxel may increase the time until docetaxel resistance in mCRPC. Phase 2 trials of weekly intravenous custirsen plus docetaxel extended median survival rates from 16.9 months to 23.8 months compared with single-agent docetaxel.67,68 Subsequent to treatment, significant decreases in clusterin levels were noted in patients treated with custirsen.67,68 A second phase 2 trial evaluating custirsen plus prednisone compared with mitoxantrone plus prednisone in patients with mCRPC who previously failed first-line docetaxel showed an increase of 4.3 months in median overall survival and a 3.8-month increase in progression-free survival as well as improved declines in PSA.52 Phase 3 trials of custirsen are ongoing (NCT01578655), although its benefits may be limited to patients expressing high levels of clusterin.69
Prostvac-VF
The use of cancer vaccines aims to generate an immune response to specific tumor antigens. The Prostvac vaccine uses a fowlpox and vaccinia platform to deliver the PSA transgene to antigen-presenting cells, which, in turn, express and present the antigen to T cells and T-cell activation.70 In addition to PSA, the vaccine has been engineered to include B7-1, ICAM-1, and LFA-3 antigen-presenting cell costimulatory molecules.71 Phase 2 trials in patients with mCRPC have shown improvements of 8 to 9 months in median survival rates.56,72 The results of these trials suggest that Prostvac offers an improvement compared with sipuleucel-T and have resulted in the initiation of a phase 3 trial (NCT01322490).
Nivolumab
Blocking the programmed cell death 1 (PD-1)/programmed death ligand 1 (PD-L1) immunosuppressive axis has received much attention in recent years. Nivolumab is a monoclonal antibody that inhibits the interaction between PD-L1 and T-cell expressed PD-1, preventing tumor-induced loss of T-cell effector function.73 In trials of melanoma, 80% of patients responded to nivolumab therapy.74 However, limited studies in CRPC have not been as promising; phase 1 studies have failed to reach objective responses and others have shown limited or lack of PD-L1 expression by CRPCs or the immune infiltrates.53,73 However, it is possible that prospective, individual patients with mCRPC with high levels of PD-L1 could benefit from nivolumab.
Ipilimumab
As cancer progresses, it can express inhibitory ligands such as B7-1, B7-2, and PD-L1 to suppress the immune system. Ipilimumab is a monoclonal antibody that inhibits T-cell–expressed cytotoxic T-lymphocyte antigen 4 from interacting with antigen-presenting cell B7-1 and B7-2 ligands but not those on tumor cells, allowing for the continued immune-mediated destruction of tumor cells. Ipilimumab has been studied in melanoma and is the only FDA-approved immune checkpoint inhibitor on the market.40 Despite encouraging results in early clinical trials, the results of a phase 3 trial of patients with mCRPC receiving bone-directed radiotherapy prior to 10 mg/kg ipilimumab or placebo revealed no significant improvement in overall survival rates.54,55 However, individual analysis of patient subsets indicated that ipilimumab may benefit men with low disease burden, thus emphasizing the importance of appropriate patient selection.16,55
Therapeutic Opportunities on the Horizon
Treatment options to extend the overall survival of patients diagnosed with mCRPC remains a major clinical challenge. Therefore, understanding the factors that drive the process of metastasis, the homing of the metastasis to organs (eg, bone), and how prostate cancer cells form life-threatening active metastases once in the bone warrants extensive research to generate new therapies to cure the disease. Although metastasis is classically thought of as a linear sequence of events beginning with the dissemination and invasion of tumor cells from the primary site and ending with proliferation at the metastatic site, recent evidence suggests that the first steps of metastasis can occur before a patient’s tumor is diagnosed (Fig 2).75 This “step 0” of the metastatic cascade results in the non-random priming of future sites of metastasis, a concept known as the “premetastatic niche.”
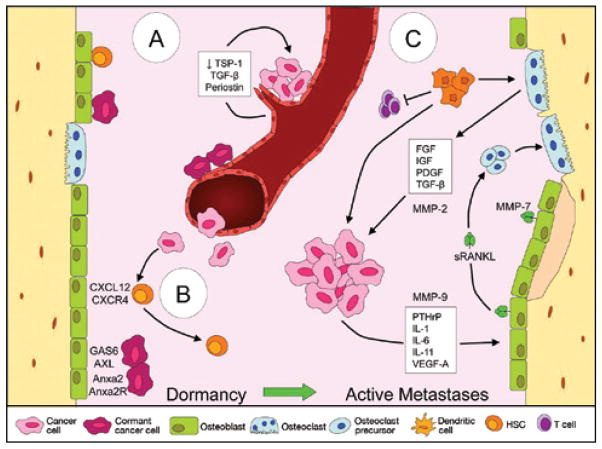
Fig 2.
A–C. Dormancy and the “vicious cycle” in bone marrow niches. (A) Disseminated tumor cells can home to the vascular niche and cluster on stable endothelium. Decreased expression of thrombospondin 1 combined with activation of transforming growth factor β and periostin in areas of “sprouting” vasculature can result in the outgrowth of tumor cells. (B) Cancer cells may also home to the endosteal niche via mechanisms such as chemokine motif 12/chemokine receptor 4 where they compete with quiescent hematopoietic stem cells for osteoblast interaction. Subsequently, the cancer cells can be maintained in a dormant state via interactions with GAS6- and ANXA2-expressing niche osteoblasts or proliferate into metastases. (C) A “vicious cycle” occurs between tumor cells and other cells of the bone microenvironment. Factors secreted by the tumor cells act on osteoblasts, leading to the increased production of RANKL. RANKL subsequently promotes the differentiation of osteoclast precursors into mature, bone-resorbing osteoclasts that degrade the bone and release additional factors into the microenvironment, providing positive feedback to the cancer cells. Matrix metalloproteinases 2, 7, and 9 contribute to the vicious cycle by regulating factors such as vascular endothelial growth factor A, RANKL, and transforming growth factor β, whereas myeloid-derived suppressor cells contribute by releasing protumorigenic factors, suppressing T cells, and differentiating into osteoclasts. RANKL = receptor activator of nuclear κB ligand.
Premetastatic Niche
Primary tumor-derived factors have been implicated in the development of premetastatic niches in distant organs.76 Through a series of in vivo experiments, it was illustrated that conditioned media derived from highly metastatic cancer cells lines, such as the B-16 melanoma cell line, could stimulate the mobilization of bone marrow–derived VEGF receptor 1+ VLA4+ Id3+ hematopoietic precursor cells to develop premetastatic niche sites, including the lungs, liver, spleen, kidney, and testes.76 Cancer-derived exosomes have been implicated as the mechanism for facilitating long distance, tumor–stroma interactions and initiating the premetastatic niche.77 Exosomes are microvesicles measuring 30 nm to 100 nm that contain a variety of functional proteins and messenger/micro RNAs.78 In the context of premetastatic niche formation, B16-F10–derived exosomes have been labeled and shown to “home” to common sites of melanoma metastasis.75 Furthermore, in the premetastatic niche, exosomes can educate bone marrow–derived cells to support metastatic tumor growth via the horizontal transfer of the c-MET protein.75 c-MET inhibitors, such as cabozantinib, could be used to prevent the development of premetastatic niches and, thus, mitigate the ability of cancers to metastasize to new sites.
Exosome shedding has also been demonstrated in prostate cancer, and studies have shown the presence of microvesicles termed oncosomes (0.5–5 μm) in prostate cancer–conditioned media. Oncosomes contain a variety of signal transduction proteins, including Akt and Src, and can interact with tumor and stromal cells to elicit disease-promoting responses.79 In addition, a correlation exists between a Gleason score higher than 7 and the number of oncosomes present in patient plasma.80 Based on these findings, it is plausible that prostate cancer–derived exosomes can play a role in the formation of premetastatic niches in the bone microenvironment. Emerging evidence also suggests that prostate cancer cells homing to the bone microenvironment can occupy the endosteal niche, the vascular niche, or both.81
Defining Factors Controlling the Homing of Bone Metastatic Castration-Resistant Prostate Cancer
An unsolved question regarding metastasis is why prostate cancer has such a predilection for the bone microenvironment. More than a century ago, Paget82 formulated the “seed and soil” hypothesis to address this question. His hypothesis suggested that metastasis is a challenging process that requires “fertile soil” for outgrowth but begins long before the “seed” meets the “soil.”82 Ewing83 challenged Paget’s hypothesis in the 1920s, proposing that metastasis was instead dependent on anatomy, vasculature, and lymphatics. Metastasis by anatomy would become the accepted model until the 1970s when modern experiments rekindled interest in the “seed and soil” hypothesis, notably observing that circulating tumor cells reach the vasculature of all organs, but only certain organs are receptive for metastasis.84,85 In reality, prostate to bone metastasis occurs by a blend of both hypotheses: It metastasizes first to the pelvic lymph node and then to sites in the bone, including iliac crests, sacrum wings, L1 to L5 vertebrae, T8 to T12 vertebrae, ribs, manubrium, humeral heads, and femoral necks.86 Although 15% to 30% of prostate to bone metastases are due to cells traveling through the Batson plexus to the lumbar spine, it is clear that molecular factors, such as chemokines and integrins, underpin the propensity for prostate cancer cells to metastasize to the skeleton.11 Elucidating those factors could help identify new therapies to prevent bone metastatic CRPC.
Bone is the home of regulatory sites for hematopoietic stem cells (HSCs), which are cells localized to the vascular and endosteal niches where they either await hematopoietic demand or reside in a quiescent state.81 One well-defined signaling axis implicated in metastasis is that between stromal cell–derived factor 1/CXCL12 and its receptor CXCR4, a system normally utilized by HSCs homing to the niche.87 CXCL12 expression is increased in the premetastatic niche, and studies in prostate cancer have demonstrated that tumor cells with high bone-homing capacity express CXCR4 and CXCR7 to parasitize the HSC niche.76,88,89 Furthermore, CXCR4 expression correlates with poor prognosis.90 Additional axes, including MCP-1/CCR2 and CXCL16/CXCR6, have also been found to contribute to the progression of prostate cancer through increases in proliferation, migration, and invasion.91,92
Disseminated Tumor Cells and Dormancy
Evidence suggests that tumor cells disseminated from the prostate localize to the bone marrow niche, displace HSCs, and either proliferate to form a metastatic mass or enter a state of dormancy.93 Dissemination from the primary site to reside in distant environments is an early event seen in prostate cancer, as patients who undergo prostatectomy may present with metastases many years later.94,95 Disseminated tumor cells (DTCs) reside in the bone marrow niche where they can remain dormant and resistant to chemotherapy for long periods of time (> 10 years) before emerging to form metastatic outgrowths.94 Although most patients with prostate cancer harbor DTCs, not all will develop metastases, suggesting that mechanisms exist to maintain DTC dormancy as well as to promote awakening.95
Several bone marrow–dependent mechanisms have been identified as modulators of prostate cancer DTC dormancy. In the endosteal niche, the osteoblast expression of Anxa2 combined with the expression of the Anxa2 receptor (Anxa2R) by HSCs is important in regulating HSC homing to the niche. Anxa2R expression is elevated in metastatic prostate tumor cells and, as such, the Anxa2/Anxa2R axis can be hijacked to promote the homing of prostate tumor cells to the niche. Interrupting the interaction between Anxa2 and Anxa2R is sufficient to reduce tumor burden in the niche.96 Evidence has revealed that the ligation of Anxa2 with Anxa2R stimulates the expression of the Axl receptor tyrosine kinase.97 Axl, along with Tyro3 and Mer, are receptors for osteoblast-expressed growth arrest-specific 6 (GAS6).98 As was the case with Anxa2/Anxa2R, the GAS6/Axl interaction typically occurs between HSCs and osteoblasts and is one mechanism of controlling HSC dormancy.98 Engaging osteoblast-expressed GAS6 and tumor cell–expressed Axl yields a similar result that includes growth arrest and enhanced drug resistance in prostate cancer cells.97 Following-up on these observations, data show that these activities may be specific to the Axl receptor compared with other GAS6 receptors.98 A high ratio of Axl to Tyro3 expression encourages maintenance of a dormant state, whereas reducing the expression of Axl and increasing the expression of Tyro3 has been shown to promote outgrowth.98
Interactions between osteoblasts and tumor cells may be important to DTC dormancy. Prostate cancer cells that bind with osteoblasts also upregulate the expression of TANK-binding kinase 1 (TBK1). In vitro and in vivo knockdown of TBK1 resulted in decreased drug resistance, suggesting that TBK1 may also play a role in dormancy and drug resistance.100 A high p38:ERK ratio has been shown to maintain dormancy of squamous carcinoma cells, whereas interactions with the micro-environment can stimulate a switch to high ERK:p38 and reverse dormancy.101 Bone marrow–derived transforming growth factor (TGF) β2 has been implicated in maintaining the dormancy of DTCs by p38 activation, and inhibiting either the TGF-β receptor 1 or p38 leads to the proliferation and metastasis of DTCs.102 Similarly, bone morphogenetic protein 7 triggers prostate cancer DTC dormancy in part by activating p38.103
Although much focus has been on the endosteal niche, the vascular niche also has implications for DTC dormancy. Through the use of advanced imaging techniques, dormant DTCs have been shown to home to perivascular niches in the bone marrow and the lungs.104 These niches promote dormancy through the expression of TSP-1; however, dormancy is lost in regions of sprouting vasculature due to a loss of TSP-1 and the activation of TGF-β and periostin.104
In vivo experiments in mice receiving bone marrow transplantation revealed that fewer HSCs successfully engraft in tumor-bearing mice, suggesting that the tumor cells occupying the niche outcompete HSCs for residence.105 In addition, expanding the endosteal osteoblast niche with parathyroid hormone (PTH) promoted metastasis, whereas decreasing the size of the niche using conditional osteoblast knockout models reduced dissemination.105 Tumor cells can also be forced out of the niche using methods to mobilize HSCs, perhaps offering an opportunity for therapeutic intervention.105 Filgrastim is an agent that mobilizes HSCs out of the niche, and plerixafor blocks the interaction with stromal cell–derived factor 1 by acting as a CXCR4 antagonist to mobilize HSCs.106 Both agents have been approved by the FDA and may serve as a method of awakening and forcing the DTCs into circulation where they would become vulnerable to chemotherapy. A small molecule inhibitor specific to CXCR6 but not other chemokine receptors was developed for investigating the CXCL16/CXCR6 axis.107 Although the clinical utility of such an inhibitor must be investigated, the selectivity of small molecule antagonists could aid in the targeting of dormant tumor cells.
Therapeutic Opportunities for “Active” mCRPC
Although therapies to prevent the homing and establishment of mCRPC in the bone microenvironment are important clinical tactics, many patients in the clinical setting present with “active” bone metastases that cause extensive bone remodeling. Defining the mechanisms that control cell–cell communication between the metastases and the microenvironment are also likely to reveal important therapeutic targets.
Osteomimicry
A recurring theme in bone metastasis is the hijacking of normal bone mechanisms by tumor cells. The concept of osteomimicry is that bone metastatic prostate cells acquire the ability to produce proteins typically restricted to bone cells, such as osteoblasts, to survive and proliferate in the otherwise restrictive bone microenvironment.108 Select genes normally expressed in bone have been detected in prostate cells, including osteocalcin, osteopontin, bone sialoprotein, osteonectin, RANK, RANKL, and PTH-related protein.108–111 The expression of these genes appears to be associated with the metastatic capacity of the cells. Studies in both the PC3 and LNCaP cell lines have shown that the expression of osteonectin is highest in the more invasive and metastatic sublines, including the LNCaP metastatic variant C4-2B.109 Analysis of patient samples support these findings, showing that osteonectin staining in prostate to bone metastases was more intense than from soft-tissue metastases.109 In addition to changes in gene expression, prostate tumor cells may adopt biological activities usually specific to bone cells. In vitro studies indicate that human C4-2B prostate tumor cells are capable of depositing hydroxyapatite and contributing to mineralization, a common feature of the sclerotic lesions observed in vivo.110
Due to the shared expression of specific bone genes between tumor and stroma cells, these common proteins could be used to simultaneously target both compartments. Understanding that soluble factors like bone morphogenetic protein 2, RANKL, TGF-β, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor are partially responsible for inducing osteomimetic genes may also provide options to specifically target osteomimicry and establish bone outgrowths.111 It has been suggested that promoters for the common genes between the tumor and stroma cells could be utilized to drive the expression of therapeutic genes, thus targeting both the stroma and tumor cells.108
Halting the Vicious Cycle of Bone Metastases
Once the DTCs awaken and establish micrometastases, continued outgrowth arises through the interaction with multiple stromal cell types, growth factors, and enzymes in a process known as the vicious cycle model.112 Prostate to bone metastases are characterized by areas of mixed osteogenesis and osteolysis that give rise to painful lesions.113 A number of tumor-derived factors, including PTH-related protein, interleukin (IL) 1, IL-6, and IL-11, have been shown to interact with osteoblasts and stimulate the production of RANKL.114 RANKL is a crucial molecule for osteoclast differentiation; therefore, it contributes to the extensive bone remodeling seen in bone metastasis. In addition to bone destruction, osteoclast-mediated bone resorption also releases a multitude of bone-derived factors such as TGF-β, insulin growth factor, platelet-derived growth factor, and fibroblast growth factor. These factors provide positive feedback via interaction with their respective receptors on the surface of tumor cells, thus promoting the proliferation and continued production of tumor-derived factors.114 The vicious cycle is continually evolving to include other cell types, cytokines, proteases, and therapeutics.115–118 Several studies have shown contributory roles for highly expressed host matrix metalloproteinases (MMPs) in the vicious cycle, including the regulation of latent TGF-β and VEGF-A bioavailability by MMP-2 and MMP-9, and the generation of a soluble form of RANKL by MMP-7, which promotes osteoclastogenesis and mammary tumor–induced osteolysis in vivo.119–121 In recent years, the interactions with immune cells have become an integral part of the vicious cycle. For example, T cells stimulate and inhibit the formation of osteoclasts, and the recruitment of regulatory T cells to bone marrow may inhibit osteoclastogenesis. Myeloid-derived suppressor cells suppress T cells and release angiogenic, tumor-promoting factors. Recruited myeloid-derived suppressor cells have also been shown to differentiate into osteoclasts.118
Although the need for therapies aimed at the early stages of metastasis has been emphasized, patients will still present in the later stages of the disease; therefore, improving therapies for these patients must still remain a priority. The interactions between tumor and stromal cells in the vicious cycle model offer many opportunities to intervene. Therapies such as zoledronic acid and denosumab interfere with the osteolytic component of the vicious cycle; however, therapies to inhibit the unique osteosclerotic component of prostate to bone metastases are lacking. Many roles for specific MMPs have been elucidated in the vicious cycle,115,120,121 and the development of MMP inhibitors with improved specificity is perhaps a promising method to modulate the vicious cycle.122
From these discoveries, it is becoming evident that the metastasis of prostate cancer is not a linear, stepwise procedure. Defining the mechanisms that control CRPC metastasis may help elucidate new therapeutic targets that directly impact the cancer cells and the processes that facilitate the formation of a premetastatic niche, niche seeding, dormancy, and the vicious cycle.123 Such new discoveries are highly likely to impact the clinical treatment of patients with mCRPC.
Upcoming Challenges
Our knowledge of the mechanisms driving the progression of prostate cancer is growing. Although several new therapies that target both the cancer cells and the supporting microenvironment and are likely to increase overall survival rates for men with mCRPC, new challenges are also emerging, particularly within the context of tumor heterogeneity. Heterogeneity is a key aspect of cancer evolution and is a clinical reality in many cancers, including prostate cancer.124–126 Greater heterogeneity facilitates the evolution of the treatment resistance of cancer but also gives the cancer a number of phenotypic strategies that allow for growth in select microenvironments (eg, bone).
Emerging studies suggest that most patients would be best served by therapies tailored toward cancer cells harboring common aberrations as well as by therapies geared toward smaller subpopulations who could potentially become the dominant-resistant population.127 The therapies described herein constitute new ways in which to expand the number of potential options for the treatment of heterogeneous bone metastatic CRPCs. However, a challenge emerging with the advent of these therapies is how to rationally design a treatment strategy for individual patients. Current guidelines from the National Comprehensive Cancer Network provide recommendations for applying the sequence of existing therapies to patients with mCRPC based on individual patient parameters. However, some studies suggest that altering the sequence or the combination of existing therapies can have a profound impact on overall survival rates.128 To circumvent costly and time-consuming clinical trials assessing the combination and sequence alterations of a new line of targeted therapies currently in clinical trials, alternative approaches are required. In this regard, integrating computational models and genetic algorithms with individual patient-derived biological data might lead to the rapid optimization of therapy choice and sequence. In the preclinical setting, the power of this integrated approach has been demonstrated. Recent studies have discovered how appropriate drug combinations guided by computational models could minimize prostate cancer progression in vivo.129 Therefore, the refinement and validation of these approaches may assist in overcoming the challenges posed by cancer heterogeneity.
Conclusions
Metastatic castration-resistant prostate cancer is an incurable disease, but the advent of new therapies, combined with an enhanced understanding of the underlying biology, suggests that significant improvement in overall survival is within reach. An increase in the number of available treatment options will be challenging from a clinical perspective with regard to patient stratification and in selecting the optimal therapy sequence, combination, or both. However, integrating computational models and genetic algorithms based on individual patient data may help overcome this challenge and allow for the delivery of individualized treatment for patients with this disease.
Acknowledgments
This work was supported in part by funding support that Dr Lynch received from the National Cancer Institute (RO1CA143094).
Footnotes
No significant relationships exist between the authors and the companies/organizations whose products or services may be referenced in this article.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 2.American Cancer Society. [Accessed October 15, 2014];Survival rates for prostate cancer. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-survival-rates.
- 3.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 4.Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8(1):12–23. doi: 10.1038/nrclinonc.2010.136. [DOI] [PubMed] [Google Scholar]
- 5.Cookson MS, Roth BJ, Dahm P, et al. American Urological Association (AUA) Castration-Resistant Prostate Cancer. Linthicum, MD: AUA; 2014. [Accessed October 15, 2014]. https://www.auanet.org/common/pdf/education/clinical-guidance/Castration-Resistant-Prostate-Cancer.pdf. [Google Scholar]
- 6.Huang X, Chau CH, Figg WD. Challenges to improved therapeutics for metastatic castrate resistant prostate cancer: from recent successes and failures. J Hematol Oncol. 2012;5:35. doi: 10.1186/1756-8722-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society. [Accessed October 15, 2014];What are the key statistics about prostate cancer? http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics.
- 8.Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91(4):718–729. doi: 10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- 9.Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 10.Crawford ED, Petrylak D. Castration-resistant prostate cancer: descriptive yet pejorative? J Clin Oncol. 2010;28(23):e408. doi: 10.1200/JCO.2010.28.7664. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin R. Neurologic complications of prostate cancer. Am Fam Physician. 2002;65(9):1834–1840. [PubMed] [Google Scholar]
- 12.American Cancer Society. [Accessed October 15, 2014];Preventing and treating prostate cancer spread to bone. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-treating-treating-pain.
- 13.Tomblyn M. The role of bone-seeking radionuclides in the palliative treatment of patients with painful osteoblastic skeletal metastases. Cancer Control. 2012;19(2):137–144. doi: 10.1177/107327481201900208. [DOI] [PubMed] [Google Scholar]
- 14.Iagaru A, Young P, Mittra E, et al. Pilot prospective evaluation of 99mTc-MDP scintigraphy, 18F NaF PET/CT, 18F FDG PET/CT and whole-body MRI for detection of skeletal metastases. Clin Nucl Med. 2013;38(7):e290–296. doi: 10.1097/RLU.0b013e3182815f64. [DOI] [PubMed] [Google Scholar]
- 15.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal N, Di Lorenzo G, Sonpavde G, et al. New agents for prostate cancer. Ann Oncol. 2014;25(9):1700–1709. doi: 10.1093/annonc/mdu038. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society. [Accessed October 15, 2014];Prednisone. http://www.cancer.org/treatment/treatmentsandsideeffects/guidetocancerdrugs/prednisone.
- 18.National Comprehensive Cancer Network. [Accessed October 15, 2014];NCCN clinical practice guidelines in prostate cancer. Version 2.2014. http://www.nccn.org.
- 19.Egan A, Dong Y, Zhang H, et al. Castration-resistant prostate cancer: adaptive responses in the androgen axis. Cancer Treat Rev. 2014;40(3):426–433. doi: 10.1016/j.ctrv.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108(33):13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizaki F, Nishiyama T, Kawasaki T, et al. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Sci Rep. 2013;3:1528. doi: 10.1038/srep01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Bono JS, Logothetis CJ, Molina A, et al. COU-AA-302 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan CJ, Smith MR, de Bono JS, et al. COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto Á. Beyond abiraterone: new hormonal therapies for metastatic castration-resistant prostate cancer. Cancer Biol Ther. 2014;15(2):149–155. doi: 10.4161/cbt.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. [Accessed October 15, 2014];Enzalutamide (XTANDI capsules) http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm317997.htm.
- 27.Scher HI, Fizazi K, Saad F, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 28.Fizazi K, Albiges L, Massard C, et al. Novel and bone-targeted agents for CRPC. Ann Oncol. 2012;23(suppl 10):x264–x267. doi: 10.1093/annonc/mds353. [DOI] [PubMed] [Google Scholar]
- 29.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate vancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Bono JS, Oudard S, Ozguroglu M, et al. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 32.Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 33.Saad F, Gleason DM, Murray R, et al. Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 34.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker C, Nilsson S, Heinrich D, et al. ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 36.Tannock IF, de Wit R, Berry WR, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 37.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 38.National Cancer Institute. FDA approval for cabazitaxel. National Cancer Institute; 2013. [Accessed October 15, 2014]. http://www.cancer.gov/cancertopics/druginfo/fda-cabazitaxel. [Google Scholar]
- 39.National Cancer Institute. [Accessed October 15, 2014];FDA approval for sipuleucel-T. http://www.cancer.gov/cancertopics/druginfo/fda-sipuleucel-T.
- 40.Schweizer MT, Drake CG. Immunotherapy for prostate cancer: recent developments and future challenges. Cancer Metastasis Rev. 2014;33(2–3):641–655. doi: 10.1007/s10555-013-9479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers MJ, Watts DJ, Russell RG. Overview of bisphosphonates. Cancer. 1997;80(8 suppl):1652–1660. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute. [Accessed October 15, 2014];FDA approval for denosumab. http://www.cancer.gov/cancertopics/druginfo/fda-denosumab.
- 43.Helo S, Manger JP, Krupski TL. Role of denosumab in prostate cancer. Prostate Cancer Prostatic Dis. 2012;15(3):231–236. doi: 10.1038/pcan.2012.2. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong AP, Miller RE, Jones JC, et al. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68(1):92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- 45.Miller RE, Roudier M, Jones J, et al. RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther. 2008;7(7):2160–2169. doi: 10.1158/1535-7163.MCT-08-0046. [DOI] [PubMed] [Google Scholar]
- 46.National Cancer Institute. [Accessed October 15, 2014];FDA approval for radium 223 dichloride. http://www.cancer.gov/cancertopics/druginfo/fda-radium-223-dichloride.
- 47.Cheetham PJ, Petrylak DP. Alpha particles as radiopharmaceuticals in the treatment of bone metastases: mechanism of action of radium-223 chloride (Alpharadin) and radiation protection. Oncology (Williston Park) 2012;26(4):330–337. 341. [PubMed] [Google Scholar]
- 48.De Wit RFK, Jinga V, Efstathiou E, et al. Phase 3, randomized, placebo-controlled trial of orteronel (TAK-700) plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC) (ELM-PC4 trial) J Clin Oncol. 2014;32(suppl 5):5008. [Google Scholar]
- 49.Dreicer R, JR, Oudard S, Efstathiou E, et al. Results from a phase 3, randomized, double-blind, multicenter, placebo-controlled trial of orteronel (TAK-700) plus prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed during or following docetaxel-based therapy (ELM-PC5 trial) J Clin Oncol. 2014;32(suppl 4):7. doi: 10.1200/JCO.2014.56.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osanto S, van Poppel H, Burggraaf J. Tasquinimod: a novel drug in advanced prostate cancer. Future Oncol. 2013;9(9):1271–1281. doi: 10.2217/fon.13.136. [DOI] [PubMed] [Google Scholar]
- 51.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saad F, Hotte S, North S, et al. Canadian Uro-Oncology Group. Randomized phase II trial of Custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c. Clin Cancer Res. 2011;17(17):5765–5773. doi: 10.1158/1078-0432.CCR-11-0859. [DOI] [PubMed] [Google Scholar]
- 53.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerritsen WR, Sharma P. Current and emerging treatment options for castration-resistant prostate cancer: a focus on immunotherapy. J Clin Immunol. 2012;32(1):25–35. doi: 10.1007/s10875-011-9595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pili R, Häggman M, Stadler WM, et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol. 2011;29(30):4022–4028. doi: 10.1200/JCO.2011.35.6295. [DOI] [PubMed] [Google Scholar]
- 58.Yamaoka M, Hara T, Hitaka T, et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J Steroid Biochem Mol Biol. 2012;129(3–5):115–128. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Agus DBSW, Shevrin DH, Hart L, et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): updated data from a phase I/II study. J Clin Oncol. 2012;30(suppl 5):98. [Google Scholar]
- 60.Jennbacken K, Welén K, Olsson A, et al. Inhibition of metastasis in a castration resistant prostate cancer model by the quinoline-3-carboxamide tasquinimod (ABR-215050) Prostate. 2012;72(8):913–924. doi: 10.1002/pros.21495. [DOI] [PubMed] [Google Scholar]
- 61.Hiratsuka S, Watanabe A, Aburatani H, et al. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8(12):1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 62.Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10(11):1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 63.Olsson A, Björk A, Vallon-Christersson J, et al. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer. 2010;9:107. doi: 10.1186/1476-4598-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scher HI, Smith MR, Sweeney C, et al. An exploratory analysis of bone scan lesion area (BSLA), circulating tumor cell (CTC) change, pain reduction, and overall survival (OS) in patients with castration-resistant prostate cancer (CRPC) treated with cabozantinib (cabo): updated results of a phase II non-randomized expansion (NRE) cohort. J Clin Oncol. 2013;31(suppl):5026. [Google Scholar]
- 65.Cochrane DR, Wang Z, Muramaki M, et al. Differential regulation of clusterin and its isoforms by androgens in prostate cells. J Biol Chem. 2007;282(4):2278–2287. doi: 10.1074/jbc.M608162200. [DOI] [PubMed] [Google Scholar]
- 66.Zellweger T, Chi K, Miyake H, et al. Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res. 2002;8(10):3276–3284. [PubMed] [Google Scholar]
- 67.Chi KN, Bjartell A, Dearnaley D, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol. 2009;56(4):594–605. doi: 10.1016/j.eururo.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 68.Chi KN, Hotte SJ, Yu EY, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(27):4247–4254. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]
- 69.Higano CS. Potential use of custirsen to treat prostate cancer. Onco Targets Ther. 2013;6:785–797. doi: 10.2147/OTT.S33077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18(7):1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peinado H, Ale kovi M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Webber J, Steadman R, Mason MD, et al. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 78.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 79.Di Vizio D, Kim J, Hager MH, et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69(13):5601–5609. doi: 10.1158/0008-5472.CAN-08-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Vizio D, Morello M, Dudley AC, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181(5):1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105(7):2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 82.Paget G. Remarks on a case of alternate partial anaesthesia. Br Med J. 1889;1(1462):1–3. doi: 10.1136/bmj.1.1462.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ewing J. Neoplastic Diseases. 6. Philadelphia: W.B. Saunders; 1928. [Google Scholar]
- 84.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 85.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 86.Roudier MP, Vesselle H, True LD, et al. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: the effect of bisphosphonate therapy on bone scintigraphy results. Clin Exp Metastasis. 2003;20(2):171–180. doi: 10.1023/a:1022627421000. [DOI] [PubMed] [Google Scholar]
- 87.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16(10):1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 88.Wang J, Loberg R, Taichman RS. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006;25(4):573–587. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- 89.Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res. 2011;71(9):3268–3277. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akashi T, Koizumi K, Tsuneyama K, et al. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99(3):539–542. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66(12):1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 92.Lu Y, Wang J, Xu Y, et al. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res. 2008;6(4):546–554. doi: 10.1158/1541-7786.MCR-07-0277. [DOI] [PubMed] [Google Scholar]
- 93.Shiozawa Y, Havens AM, Pienta KJ, et al. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22(5):941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vessella RL, Pantel K, Mohla S. Tumor cell dormancy: an NCI workshop report. Cancer Bio Ther. 2007;6(9):1496–1504. doi: 10.4161/cbt.6.9.4828. [DOI] [PubMed] [Google Scholar]
- 95.Lam HM, Vessella RL, Morrissey C. The role of the microenvironment-dormant prostate disseminated tumor cells in the bone marrow. Drug Discov Today Technol. 2014;11:41–47. doi: 10.1016/j.ddtec.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shiozawa Y, Havens AM, Jung Y, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105(2):370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shiozawa Y, Pedersen EA, Patel LR, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taichman RS, Patel LR, Bedenis R, et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One. 2013;8(4):e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dormady SP, Zhang XM, Basch RS. Hematopoietic progenitor cells grow on 3T3 fibroblast monolayers that overexpress growth arrest-specific gene-6 (GAS6) Proc Natl Acad Sci U S A. 2000;97(22):12260–12265. doi: 10.1073/pnas.97.22.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JK, Jung Y, Wang J, et al. TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia. 2013;15(9):1064–1074. doi: 10.1593/neo.13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bragado P, Sosa MS, Keely P, et al. Microenvironments dictating tumor cell dormancy. Recent Results Cancer Res. 2012;195:25–39. doi: 10.1007/978-3-642-28160-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bragado P, Estrada Y, Parikh F, et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38β/β signalling. Nat Cell Biol. 2013;15(11):1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kobayashi A, Okuda H, Xing F, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208(13):2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pedersen EA, Shiozawa Y, Pienta KJ, et al. The prostate cancer bone marrow niche: more than just ‘fertile soil’. Asian J Androl. 2012;14(3):423–427. doi: 10.1038/aja.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hershberger PM, Peddibhotla S, Sugarman E, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda, MD: National Center for Biotechnology Information; 2012. Probing the CXCR6/CXCL16 Axis: targeting prevention of prostate cancer metastasis. [PubMed] [Google Scholar]
- 108.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39(4):246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 109.Thomas R, True LD, Bassuk JA, et al. Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res. 2000;6(3):1140–1149. [PubMed] [Google Scholar]
- 110.Lin DL, Tarnowski CP, Zhang J, et al. Bone metastatic LNCaP-derivative C4-2B prostate cancer cell line mineralizes in vitro. Prostate. 2001;47(3):212–221. doi: 10.1002/pros.1065. [DOI] [PubMed] [Google Scholar]
- 111.Chu GC, Chung LW. RANK-mediated signaling network and cancer metastasis. Cancer Metastasis Rev. 2014;33(2–3):497–509. doi: 10.1007/s10555-013-9488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 suppl):1546–1556. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 113.Roudier MP, Morrissey C, True LD, et al. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180(3):1154–1160. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 115.Lynch CC. Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone. 2011;48(1):44–53. doi: 10.1016/j.bone.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 116.Faccio R. Immune regulation of the tumor/bone vicious cycle. Ann N Y Acad Sci. 2011;1237:71–78. doi: 10.1111/j.1749-6632.2011.06244.x. [DOI] [PubMed] [Google Scholar]
- 117.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310(1–2):71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 118.Cook LM, Shay G, Aruajo A, et al. Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metastasis Rev. 2014;33(2–3):511–525. doi: 10.1007/s10555-014-9494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lynch CC, Vargo-Gogola T, Martin MD, et al. Matrix metalloproteinase 7 mediates mammary epithelial cell tumorigenesis through the ErbB4 receptor. Cancer Res. 2007;67(14):6760–6767. doi: 10.1158/0008-5472.CAN-07-0026. [DOI] [PubMed] [Google Scholar]
- 120.Thiolloy S, Edwards JR, Fingleton B, et al. An osteoblast-derived proteinase controls tumor cell survival via TGF-beta activation in the bone microenvironment. PLoS One. 2012;7(1):e29862. doi: 10.1371/journal.pone.0029862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bruni-Cardoso A, Johnson LC, Vessella RL, et al. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Mol Cancer Res. 2010;8(4):459–470. doi: 10.1158/1541-7786.MCR-09-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tauro M, McGuire J, Lynch CC. New approaches to selectively target cancer associated matrix metalloproteinase activity. Cancer Metastasis Rev. 2014 doi: 10.1007/s10555-014-9530-4. In press. [DOI] [PubMed] [Google Scholar]
- 123.Esposito M, Kang Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol Ther. 2014;141(2):222–233. doi: 10.1016/j.pharmthera.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing [Erratum appears in N Engl J Med. 2012;367(10):976] N Engl J Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gerlinger M, Catto JW, Orntoft TF, et al. Intratumour heterogeneity in urologic cancers: from molecular evidence to clinical implications. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.04.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 126.Drake JM, Graham NA, Lee JK, et al. Metastatic castration-resistant prostate cancer reveals intrapatient similarity and interpatient heterogeneity of therapeutic kinase targets. Proc Natl Acad Sci U S A. 2013;110(49):E4762–E4769. doi: 10.1073/pnas.1319948110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gallaher J, Cook LM, Gupta S, et al. Improving treatment strategies for patients with metastatic castrate resistant prostate cancer through personalized computational modeling. Clin Exp Metastasis. 2014 doi: 10.1007/s10585-014-9674-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sweeney C, Chen YH, Carduccie MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase 3 randomized trial. J Clin Oncol. 2014;32(5 suppl):LBA2. [Google Scholar]
- 129.Zhao B, Pritchard JR, Lauffenburger DA, et al. Addressing genetic tumor heterogeneity through computationally predictive combination therapy. Cancer Discov. 2014;4(2):166–174. doi: 10.1158/2159-8290.CD-13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]