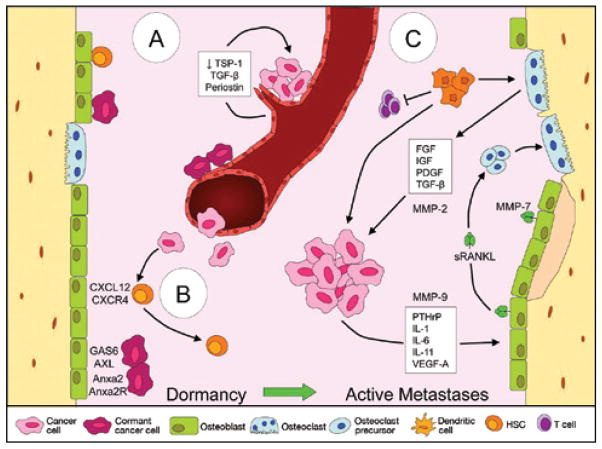
Fig 2.
A–C. Dormancy and the “vicious cycle” in bone marrow niches. (A) Disseminated tumor cells can home to the vascular niche and cluster on stable endothelium. Decreased expression of thrombospondin 1 combined with activation of transforming growth factor β and periostin in areas of “sprouting” vasculature can result in the outgrowth of tumor cells. (B) Cancer cells may also home to the endosteal niche via mechanisms such as chemokine motif 12/chemokine receptor 4 where they compete with quiescent hematopoietic stem cells for osteoblast interaction. Subsequently, the cancer cells can be maintained in a dormant state via interactions with GAS6- and ANXA2-expressing niche osteoblasts or proliferate into metastases. (C) A “vicious cycle” occurs between tumor cells and other cells of the bone microenvironment. Factors secreted by the tumor cells act on osteoblasts, leading to the increased production of RANKL. RANKL subsequently promotes the differentiation of osteoclast precursors into mature, bone-resorbing osteoclasts that degrade the bone and release additional factors into the microenvironment, providing positive feedback to the cancer cells. Matrix metalloproteinases 2, 7, and 9 contribute to the vicious cycle by regulating factors such as vascular endothelial growth factor A, RANKL, and transforming growth factor β, whereas myeloid-derived suppressor cells contribute by releasing protumorigenic factors, suppressing T cells, and differentiating into osteoclasts. RANKL = receptor activator of nuclear κB ligand.