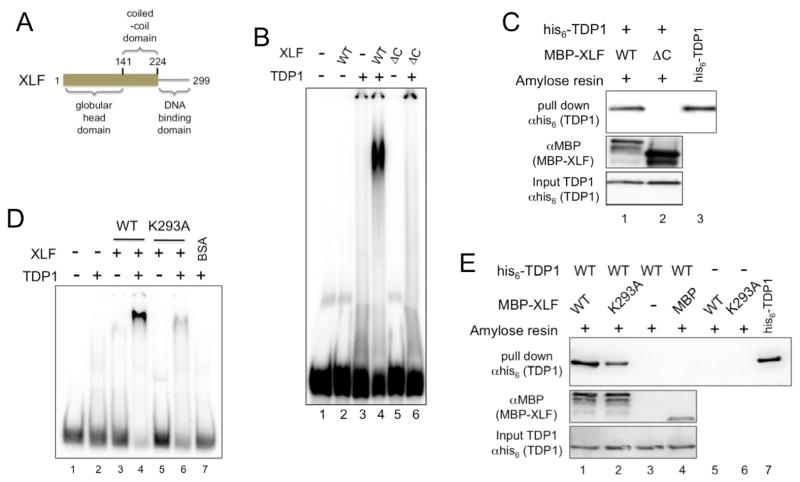
Fig. 3. DNA binding by XLF is required for XLF:TDP1 interactions.
(A) Schematic diagram of XLF.
(B) C-terminally truncated XLF fails to bind DNA in the presence of TDP1. EMSA was carried out as described in materials and methods using 100 ng TDP1 and 300 ng of wild type XLF (WT) or C-terminally truncated XLF mutant XLF 1-224 (ΔC).
(C) C-terminally truncated XLF does not interact with TDP1. MBP-XLF, or XLF 1-224 (ΔC) were used to pull down wild type his6-TDP1. Samples were separated by SDS-PAGE and Western transferred. His6-TDP1 proteins were detected in the pull-down eluates (top) and input (bottom) using anti-his6 monoclonal antibodies. MBP-XLF proteins were detected with anti-MBP polyclonal antibodies (center). Lane 3, his6-TDP1 protein positive control for Western blot.
(D) XLF DNA-binding mutant XLF-K293A fails to bind DNA in the presence of TDP1. EMSA was carried out as described in materials and methods using 100 ng TDP1 and 300 ng of wild type XLF (WT), mutant XLF-K293A (K293A) or BSA.
(E) DNA-binding mutant XLF-K293A interacts with TDP1. MBP-XLF, or XLF-K293A (K293A), were used to pull down wild type his6-TDP1 on amylose resin. Samples were separated by SDS-PAGE and Western transferred. His6-TDP1 proteins were detected in the pull-down eluate (top) and input (bottom) samples using anti-his6 monoclonal antibodies. MBP-XLF and MBP proteins were detected with anti-MBP polyclonal antibodies (center). Lane 7, his6-TDP1 protein positive control for Western blot.