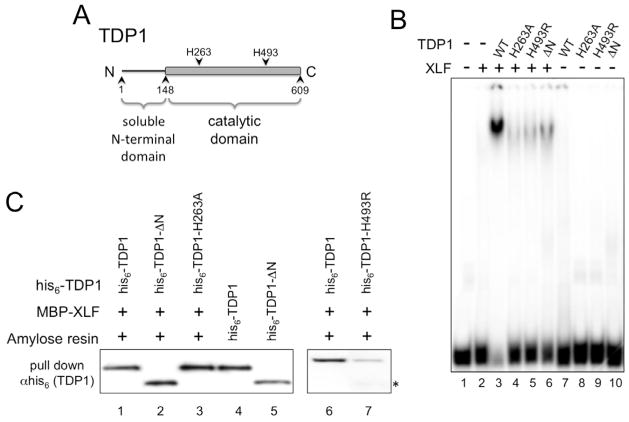
Fig. 4. DNA binding by TDP1 is required for TDP1:XLF:DNA interaction but not for TDP1:XLF physical interaction.
(A) Schematic diagram of TDP1.
(B) Active site, SCAN1 and N-terminal deletion mutants of TDP1 do not bind DNA in the presence of XLF. EMSA was carried out as described in materials and methods using 300 ng of XLF and 100 ng of wild type TDP1 (WT), active site mutant TDP1-H236A (H263A), SCAN1 mutant TDP1-H493R (H493R) or N-terminally truncated TDP1-ΔN (ΔN).
(C) Physical interactions between active site and N-terminal truncation mutants of TDP1 and XLF. MBP-XLF was used to pull down wild type his6-TDP1, N-terminally truncated his6-TDP1-ΔN and active site mutants his6-TDP1-H236A and -H493R. Samples were separated by SDS-PAGE, Western transferred and his-tagged TDP1 proteins were detected in the pull-down eluates using anti-his6 monoclonal antibodies. Lanes 4 and 5, his6-TDP1 and his6-TDP1-ΔN positive controls for Western blot. * indicates C-terminally truncated form of TDP1-H493R that can interact with XLF