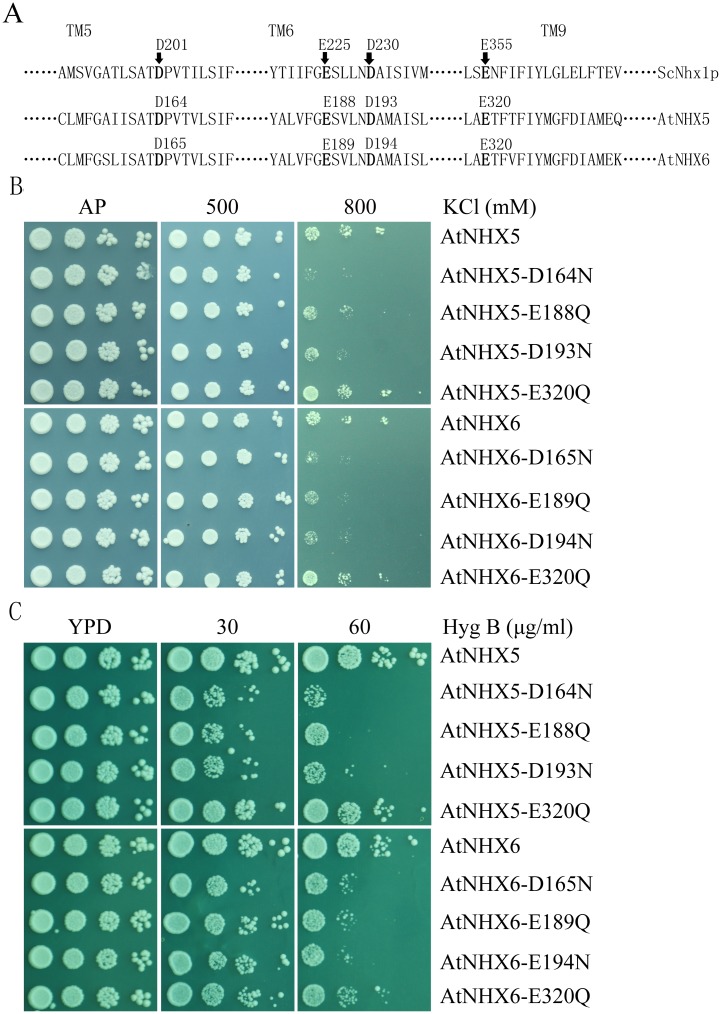
Fig 3. Three conserved acidic residues in AtNHX5 and AtNHX6 are critical for K+ transport in yeast.
(A) Sequence alignment of AtNHX5 and AtNHX6 with yeast (S. cerevisiae) ScNhx1p identified four conserved acidic amino acids in the transmembrane domains of AtNHX5 and AtNHX6. The alignment was made by using the complete amino acid sequences, but only the predicted transmembrane domains 5, 6, and 9 are shown. (B) and (C) Yeast growth test for the point mutants of AtNHX5 and AtNHX6. The yeast strains were grown overnight in AP medium. Yeast cells were normalized in water to A600 of 0.12. Aliquos (4 μL) of 10-fold serial dilutions were spotted on AP plates supplemented with KCl (B) or on YPD plates with Hyg B (C). The strains were grown at 30°C for 3 days.