Table 2.
In Vivo pharmakokinetic parameters of inhibitors in female Sprague-Dawley rats (n = 3, mean ± SD) following a single intravenous (i.v.) and oral (p.o.) administration
| Compound |
i.v. PK parametersa |
p.o. PK parametersb |
||||
|---|---|---|---|---|---|---|
| AUC0-inf (μg.min.mL–1) | CL (mL.min–1.kg–1) | t1/2 (min) | Cmax (μg.mL–1) | AUC0-inf (μg.min.mL–1) | F (%) | |
| 10c | 519 ± 115 | 4.9 ± 0.9 | 11.2 ± 2.2 | 0.40 ± 0.20 | 66 ± 41 | 1.3 ± 0.7 |
| 11c | 304 ± 60 | 8.4 ± 1.9 | 10.6 ± 0.7 | 0.70 ± 0.07 | 100 ± 14 | 3.5 ± 0.2 |

|
1040 ± 330 | 2.5 ± 0.9 | 32.5 ± 7.4 | 0.46 ± 0.30 | 187 ± 11 | 1.8 ± 0.9 |
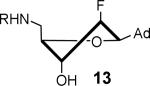
|
913 ± 65 | 2.7 ± 0.2 | 31.7 ± 1.9 | 1.09 ± 0.03 | 422 ± 70 | 4.7 ± 1.1 |
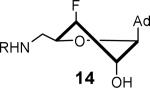
|
366 ± 33 | 6.9 ± 0.3 | 17.4 ± 1.4 | 0.64 ± 0.22 | 84 ± 12 | 2.3 ± 0.4 |
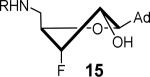
|
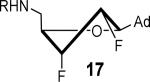
807 ± 78 | 3.1 ± 0.3 | 37.0 ± 1.7 | 0.92 ± 0.04 | 266 ± 2 | 3.5 ± 0.2 |
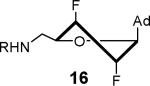
|
312 ± 54 | 8.5 ± 1.6 | 17.2 ± 1.3 | 0.97 ± 0.42 | 362 ± 85 | 11.4 ± 0.3 |
|
10100 ± 2200 | 0.24 ± 0.06 | 267 ± 36 | 5.99 ± 0.56 | 4730 ± 540 | 5.3 ± 1.3 |
i.v. dose (Di.v.) = 2.5 mg/kg
p.o. dose (Dp.o.) = 25 mg/kg
reference 34.
AUC0-inf: area under the plasma concentration-time curve from time 0 to infinity, CL: clearance, t1/2: terminal elimination half-life, Cmax: maximum plasma concentration, F: relative oral bioavailability calculated as follows F = 100 × [(AUCp.o. × Di.v.)/(AUCi.v. × Dp.o.)].
