Abstract
Melioidosis is a bacterial infection caused by Burkholderia pseudomallei, a gram-negative saprophytic bacillus. Cases occur sporadically in the Americas with an increasing number of cases observed among people with no travel history to endemic countries. To better understand the incidence of the disease in the Americas, we reviewed the literature, including unpublished cases reported to the Centers for Disease Control and Prevention. Of 120 identified human cases, occurring between 1947 and June 2015, 95 cases (79%) were likely acquired in the Americas; the mortality rate was 39%. Burkholderia pseudomallei appears to be widespread in South, Central, and North America.
Introduction
Cases of melioidosis are commonly seen in tropical and subtropical areas.1 Burkholderia pseudomallei, formerly known as Whitmore bacillus, Pseudomonas pseudomallei, or Malleomyces pseudomallei, is the causative agent of melioidosis.2 Infection most frequently occurs through contact with soil and water in endemic areas through inhalation, inoculation, or ingestion,3 with increasing evidence of ingestion playing a greater role in infection than historically thought.4,5 Infections may also occur after laboratory exposures when enhanced precautions such as those used in biosafety level 3 facilities are not used during testing of isolates from infected patients or animals.6–8 The federal select agent program designates B. pseudomallei as a Tier 1 overlap select agent.9 Melioidosis is not nationally notifiable in the United States.10,11 Thus, Centers for Disease Control and Prevention (CDC) receives reports on cases of melioidosis from state health departments, medical facilities, microbiologic laboratories, and research facilities on a voluntary basis.
Cases have been reported in both humans and animals.3 Melioidosis infections in sheep, goats, swine, cattle, primates, horses, dogs, cats, and wildlife species are well documented.12–20 Although human-to-human transmission is rare, one reported patient most likely acquired the infection through sexual transmission.21 In addition, testing of soil samples or water in Peru,22 Brazil,22–24 Haiti,22 Venezuela,25 Ecuador,25 and Puerto Rico26 has revealed the presence of B. pseudomallei in the environment in these locations. In one study, B. pseudomallei was recovered from environmental specimens (soap, floor, and shower hose) in a burn unit of a hospital in Belo Horizonte, Brazil.27
A prior review of melioidosis cases in the Americas described 105 cases with an association (residence when diagnosed, travel history, or country of birth) to the Americas.28 Two of the cases (one case reported from Oklahoma in 1976 and the other reported from Georgia in 1979) have since been reclassified as infections with the closely related bacteria Burkholderia oklahomensis.29 An additional 84 cases reported in that series occurred in soldiers/veterans with a history of service in Vietnam. Another review reported more than 340 cases among U.S. soldiers with service to Vietnam.30,31 Our review focuses on B. pseudomallei infections acquired in the Americas.
Materials and Methods
Search strategy.
We conducted a literature review of human cases diagnosed with melioidosis with residence, travel history, or country of birth associated with the Americas, including the United States and its territories. In addition, we reviewed all case reports voluntarily submitted by states to the CDC between January 2008 and December 2013.
We first conducted a search of seven databases from their inception to October 2013: PubMed of the National Library of Medicine (1996), Web of Science (1980), Excerpta Medica Databases (1974), Global Health (1910), Latin American and Caribbean Health Sciences Literature (1982), and Google Scholar (2004). Keywords used included combinations of “melioidosis,” “Burkholderia pseudomallei,” “Western Hemisphere,” “America,” and “Caribbean.”
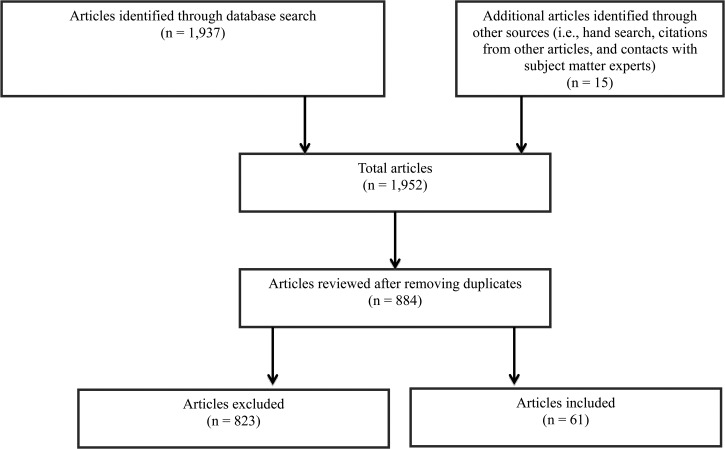
In June 2015, we conducted a broader search of case reports, meta-analyses, reviews, scientific integrity reviews, and systematic reviews from the same databases using combinations of keywords such as “Burkholderia pseudomallei,” “Pseudomonas pseudomallei,” “Whitmore bacillus,” “melioidosis,” and “Malleomyces pseudomallei.” There were no geographic limiters, language, gender, age, or dates applied to this second search strategy. Our search strategies resulted in a total of 1,937 articles. An additional 15 articles were identified through hand searching, citations from other articles, and correspondence with subject matter experts. After deduplication, the remaining 884 articles were evaluated for relevancy. Final selection of articles was based on the following criteria: description or review of a case or cases diagnosed in the Americas, or with travel history in the Americas, or residence in the Americas. A total of 61 articles (from 1945 to 2015) met our inclusion criteria (Figure 1 ). Although the majority of selected articles were published in English, four articles were published in French, two in Portuguese, and one in Spanish. Articles in French were translated by author Tina J. Benoit. Abstracts and case summaries published in Portuguese were already translated into English by the journal. A summary of a case published in Spanish was also already translated into English by the journal. Summaries of two cases published in Spanish were translated to English by a CDC Spanish speaker senior epidemiologist. All confirmed and probable cases were included in this review. Confirmed cases were defined as having a positive culture or isolation of B. pseudomallei from any clinical specimen. Probable cases were defined as having a positive result for B. pseudomallei polymerase chain reaction or a 4-fold rise in B. pseudomallei antibody titer (indirect hemagglutination assay [IHA]) between acute and convalescent samples taken 2 weeks apart. A library of all relevant references (including articles identified through other sources) was built using EndNote X6 software (www.EndNote.com) for bibliographic management.
Figure 1.
Flow chart of complex literature review of melioidosis cases in the Americas.
Data abstraction.
The following data were abstracted from each article when available: year of diagnosis, gender, age, race, occupation, country of diagnosis, diagnostic laboratory results, travel history and/or country of birth of the patient, signs and symptoms, preexisting medical conditions, risk factors, possible exposures, treatment, and patient outcomes. The year of diagnosis was documented as the year of publication of the article if such information was not reported in the article. For cases series, the year of diagnosis was documented as unknown in the online Supplemental Appendix 1.
Data analysis, map, and graphs.
The IBM Statistical Package for Social Sciences (SPSS) version 19 for Windows (Armonk, NY) was used for data analysis. ArcGIS ArcMAP version 10.2.1 (ESRI, Redlands, CA) was used to generate the map (Figure 2 ).
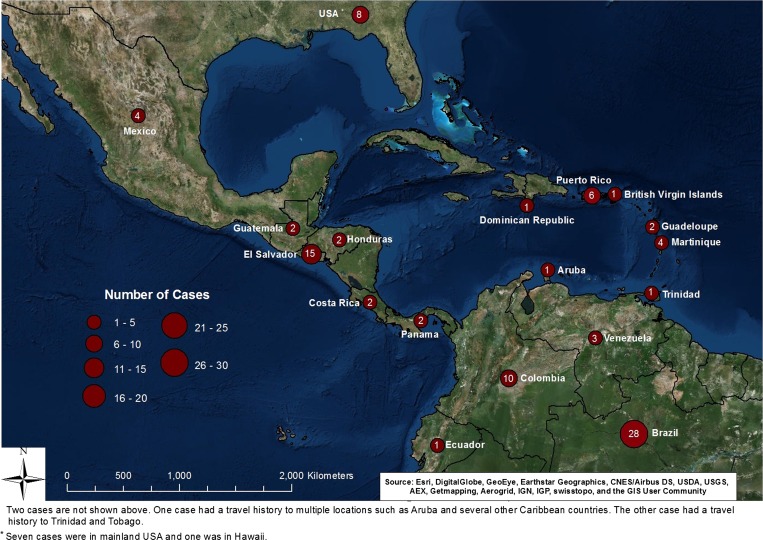
Figure 2.
Distribution of melioidosis cases in the Americas.+
Classification of countries in the Americas.
The U.S. Central Intelligence Agency 2004 World Factbook was used as a reference guide in classifying countries into North, Central, and South America.32
Results
Of 120 cases reviewed (Supplemental Appendix 1), 95 cases (79%) were believed to have had acquired the infection in the Americas (Table 1, Figure 2). The remaining 25 cases (Table 2) were either born in or had travel history to melioidosis-endemic countries.
Table 1.
Number of cases in the Americas by classification of countries of likely exposure or acquisition, 1947–2015
| Number of cases | |
|---|---|
| North America | |
| United States (mainland)* | 7 |
| Mexico | 4 |
| Noncontiguous United States (Hawaii) | 1 |
| Total | 12 |
| Central America and the Caribbean | |
| El Salvador | 15 |
| United States (Puerto Rico) | 6 |
| Martinique | 4 |
| Costa Rica | 2 |
| Guadeloupe | 2 |
| Guatemala | 2 |
| Honduras | 2 |
| Panama | 2 |
| Aruba | 1 |
| Aruba or other Caribbean countries† | 1 |
| British Virgin Islands | 1 |
| Dominican Republic | 1 |
| Trinidad | 1 |
| Trinidad or Tobago§ | 1 |
| Total | 41 |
| South America | |
| Brazil | 28 |
| Colombia | 10 |
| Venezuela | 3 |
| Ecuador | 1 |
| Total | 42 |
| Grand Total | 95 |
Two cases with likely occupational exposure in the United States, five cases acquired elsewhere in the Americas.
Patient with travel history to multiple locations such as Aruba and several Caribbean countries.
Patient with travel history to Trinidad and Tobago.
Table 2.
Number of cases with likely exposures outside the Americas
| Countries | Number of cases | Percent |
|---|---|---|
| Thailand | 3 | 12 |
| Vietnam | 3 | 12 |
| Philippines | 3 | 12 |
| Cambodia | 2 | 8 |
| India | 2 | 8 |
| Malaysia | 2 | 8 |
| Africa | 1 | 4 |
| China | 1 | 4 |
| Southeast Asia | 1 | 4 |
| Hong Kong, Vietnam, Japan, Mexico, or the Philippines | 1 | 4 |
| Indonesia | 1 | 4 |
| Laos or Cambodia | 1 | 4 |
| Laos or Thailand | 1 | 4 |
| Saudi Arabia or Bangladesh | 1 | 4 |
| Singapore, Malaysia, Burma, or Thailand | 1 | 4 |
| Vietnam, southeast and central Asia, Iraq, or Afghanistan | 1 | 4 |
| Total | 25 | 100 |
Among those cases with reported source of exposure to B. pseudomallei in the Americas (47 of 95 cases), approximately 20% reported contact with soil only, 7% reported contact with water only, and 17% reported contact with both, soil and water (Table 3). This is consistent with other reports from endemic regions.
Table 3.
Distribution of exposure sources among the 47 cases in the Americas with reported risks, 1945–2015
| Frequency | Percent | |
|---|---|---|
| Soil | 19 | 20 |
| Water | 7 | 7 |
| Soil and water | 16 | 17 |
| Animals | 2 | 2 |
| Sexual contact | 1 | 1 |
| In utero | 1 | 1 |
| Occupational exposure | 1 | 1 |
| Total | 47 | 100 |
North America.
Twelve cases were likely acquired in either the U.S. mainland (7 cases), Hawaii (1 case) or Mexico (4 cases). Two of the U.S. mainland cases potentially acquired the infection through occupational exposure. One individual worked with B. pseudomallei in a laboratory.6 The other individual, a zoological warehouse employee, may have acquired the infection through contact with reptiles (case no. 76 in the online Supplemental Appendix 1; CDC, unpublished data), as melioidosis has been reported in iguanas sold in the United States.33 An African–American infant diagnosed soon after birth likely acquired the infection in utero. The infant's father was a Vietnam veteran with unknown disease status.34 A fourth individual may have acquired infection through sexual transmission. The patient's husband was a U.S. veteran with travel history to Hong Kong, Japan, Mexico, and the Philippines.21 The source of infection for the other three cases from U.S. mainland,35–37one case from Hawaii,38 and all four cases from Mexico (Supplemental Appendix 1) remains unknown.
Central America and the Caribbean.
Cases have been reported from every country in Central America, except Belize and Nicaragua. El Salvador, Puerto Rico, and Martinique have the highest number of cases in Central America and the Caribbean (Table 1).
Five of six cases from Puerto Rico reported exposure to environmental sources such as soil, flood water, or both, while one source of exposure was unknown. None of the six had traveled outside Puerto Rico or the U.S. mainland. Most cases (13 of 15 cases) from El Salvador were Salvadoran residents and reported never traveling outside the country. The authors also did not report the year of diagnosis for those cases. Two patients that likely acquired disease in El Salvador were U.S. residents. One patient was from a 47-year-old U.S. born male with travel history to El Salvador 3 weeks before diagnosis. The other patient was a 37-year-old Salvadoran female resident of the United States and believed to have acquired infection in El Salvador.
Two of the four patients from Martinique reported no travel history outside Martinique and thus likely acquired the infection there. A third individual, a 66-year-old Martinique resident had traveled to non-endemic areas of Africa and South America more than 40 years before diagnosis. The authors reported that the patient never lived in rural areas and that the source of infection remained unknown.39 The last patient was a resident of Switzerland who became ill shortly after a 10-day visit to Martinique.40
South America.
Thirty-seven cases from 1962 to 2015 with suspected origin in South America were reported from Brazil, Colombia, Venezuela, and Ecuador. Brazil accounted for the majority (67%) of South American cases (Table 1), with the State of Ceará in northeast Brazil accounting for 91% of cases reported in Brazil.23,41 Since 2003, there have been 25 cases of melioidosis, including one family cluster, reported in Brazil. This cluster was reported among four siblings, of which three died, from a rural area of Ceará following contact with water after the rainy season.41,42
Discussion
Historically, melioidosis has been described as endemic in southeast (SE) Asia, south Asia, and northern Australia. However, our review of the literature indicates the need to update the epidemiology of melioidosis and expand the list of countries with sporadic occurrence or that may be considered endemic. Since the last review of melioidosis in the Americas,28 more than 2 dozen cases have been identified in patients with no travel history outside their native country in the Americas or in patients with travel history only within the Americas. The increasing occurrence of melioidosis in Brazil, as well as reports of the disease acquired in Central and North America suggests the disease is endemic in many countries throughout the Americas. The environmental conditions present in many of these countries, as well as environmental isolates of B. pseudomallei, support the possibility of endemicity in these areas.26
A bit more puzzling are the three cases reported from the United States with no travel history outside the U.S. mainland.35–37 One was reported as a chronic case.35 In the other two36,37 cases, extensive investigations took place, including detailed travel history, occupational history, and recreation and hobbies; in addition, environmental sampling around the home environment was carried out and serology was conducted on family members and pets. In both cases, no source of B. pseudomallei was identified and there was no serologic evidence of exposure in family members or pets. However, both patients had been hospitalized for several weeks before identification of B. pseudomallei, and strain types were consistent with SE Asian origin. The most recent case in an Ohio resident was found to be internal transcribed spacer (ITS) type CE, which is commonly seen in SE Asia and Australia but not in the Western Hemisphere.37,43,44 Since melioidosis is not considered endemic to the United States (mainland),45 it raises the question of whether contaminated medical supplies (intravenous supplies, medications, etc.) could have been imported from an endemic area, leading to a nosocomial acquisition of B. pseudomallei. However, B. pseudomallei could also have been introduced through plants or other commodities imported from endemic areas. Cases are more likely imported by travelers, animals, or environmentally contaminated materials from endemic countries.
The increasing number of cases seen with acquisition in the Americas since the first review of melioidosis in the Americas by Inglis and others28 raises the question of why this disease is being seen with increasing frequency. Specifically, does the epidemiology of melioidosis in the Americas differ from that elsewhere, or are the increasing numbers a reflection of increased awareness of this disease? Gee and others44 have noted that ITS type G is found predominantly in Western Hemisphere isolates of B. pseudomallei, and is rarely seen outside the Western Hemisphere. This could support the theory that melioidosis exhibits a different clinico-epidemiologic presentation in the Americas. However, our review found no evidence of any differences epidemiologically in the cases reported or with underlying clinical conditions. Mortality rates are similar to that seen elsewhere where melioidosis is endemic. We therefore feel that the increasing numbers seen are a reflection of increased awareness and identification of melioidosis among clinicians and laboratories.
This review has the major limitation of relying solely on peer-reviewed and published case reports, which sometimes lacked detailed information on source of exposure. Additional unpublished cases were mentioned during our review, and we have no doubt that many cases have gone either unpublished or unidentified because of lack of familiarity with B. pseudomallei by clinicians and laboratory staff. Also worth noting is that melioidosis is not a nationally reportable disease in the United States, so even cases presumptively identified at the local level may not be reported to the CDC or other public health agencies. Furthermore, many of the published case reports reviewed did not contain travel history. Finally, many of the published reports lacked information on underlying medical illness/risk factors and exposure history, limiting our ability to investigate clinico-epidemiologic patterns in more detail. With these limitations noted, this report is a more comprehensive review of melioidosis cases with presumed acquisition of infection in the Americas, providing an update to the seminal publication by Inglis and others and contributing to better understanding of the global and regional distribution of melioidosis.
Continued improvement in the accurate and timely diagnosis of melioidosis cases is crucial to understanding the incidence and epidemiology of the disease in the Americas. The early identification of B. pseudomallei infection and the initiation of appropriate antimicrobial treatment will also reduce morbidity and mortality of melioidosis. Recent publications have addressed current diagnostics and future directions46; in the meantime, physicians and laboratory personnel should be sensitized to the presence of melioidosis in the Americas. In addition, areas with clusters of melioidosis, such as in the State of Ceará, may consider additional studies, such as community serosurveys to delineate endemic areas and to allow health-care systems to focus resources where they are needed to address this emerging disease.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the work of Marta Guerra for assistance with translating Spanish articles. We also thank Marissa Person for generating the map.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the U.S. Department of Health and Human Services.
Footnotes
Authors' addresses: Tina J. Benoit, David D. Blaney, Jay E. Gee, Mindy G. Elrod, Alex R. Hoffmaster, William A. Bower, and Henry T. Walke, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: josanabenoit@hotmail.com, dblaney@cdc.gov, xzg4@cdc.gov, mglass@cdc.gov, ahoffmaster@cdc.gov, wbower@cdc.gov, and hwalke@cdc.gov. Thomas J. Doker, Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: thomas.doker@gmail.com. Dionne B. Rolim, Universidade de Fortaleza (UNIFOR), Fortaleza, Brazil, E-mail: dionnerolim@gmail.com. Timothy J. J. Inglis, School of Pathology and Laboratory Medicine, University of Western Australia, Western Australia, Australia, E-mail: tim.inglis@uwa.edu.au.
References
- 1.White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limmathurotsakul D, Wongsuvan G, Aanensen D, Ngamwilai S, Saiprom N, Rongkard P, Thaipadungpanit J, Kanoksil M, Chantratita N, Day NPJ, Peacock S. Melioidosis caused by Burkholderia pseudomallei in drinking water, Thailand, 2012. Emerg Infect Dis. 2014;20:265–268. doi: 10.3201/eid2002.121891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limmathurotsakul D, Kanoksil M, Wuthiekanun V, Kitphati R, deStavola B, Day NPJ, Peacock S. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: a matched case-control study. PLoS Negl Trop Dis. 2013;7:e2072. doi: 10.1371/journal.pntd.0002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlech WF, Turchik JB, Westlake RE, Klein GC, Band JD, Weaver RE. Laboratory-acquired infection with Pseudomonas pseudomallei (melioidosis) N Engl J Med. 1981;305:1133–1135. doi: 10.1056/NEJM198111053051907. [DOI] [PubMed] [Google Scholar]
- 7.Green RN, Tuffnell PG. Laboratory acquired melioidosis. Am J Med. 1968;44:599–605. doi: 10.1016/0002-9343(68)90060-0. [DOI] [PubMed] [Google Scholar]
- 8.Peacock SJ, Schweizer HP, Dance DA, Smith TL, Gee JE, Wuthiekanun V, DeShazer D, Steinmetz I, Tan P, Currie BJ. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis. 2008;14:e2. doi: 10.3201/eid1407.071501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federal Select Agent Program. 2014. http://www.selectagents.gov/ Available at. Accessed May 18, 2015.
- 10.Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onweh DH, Schley AW, Anderson WJ, Grigoryan A, Aranas AE, Wodajo MS, Abellera JP. Summary of notifiable diseases—United States. MMWR. 2012;61:1–121. [PubMed] [Google Scholar]
- 11.Council of State and Territorial Epidemiologists National Surveillance Definition for Melioidosis. 2011. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/11-ID-16rev.pdf Available at. Accessed May 18, 2015.
- 12.Retnasabapathy A, Joseph PG. A case of melioidosis in a macaque monkey. Vet Rec. 1966;79:72–73. doi: 10.1136/vr.79.3.72. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann AF, Alexander AD, Allen MA, Cronin RJ, Dillingham LA, Douglas JD, Moore TD. Melioidosis in imported non-human primates. J Wildl Dis. 1970;6:211–219. doi: 10.7589/0090-3558-6.4.211. [DOI] [PubMed] [Google Scholar]
- 14.Fritz PE, Miller JG, Slayter M, Smith TJ. Naturally occurring melioidosis in a colonized rhesus monkey (Macaca mulatta) Lab Anim. 1986;20:281–285. doi: 10.1258/002367786780808749. [DOI] [PubMed] [Google Scholar]
- 15.Dance DA, King C, Aucken H, Knott CD, West PG, Pitt TL. An outbreak of melioidosis in imported primates in Britain. Vet Rec. 1992;130:525–529. doi: 10.1136/vr.130.24.525. [DOI] [PubMed] [Google Scholar]
- 16.Sprague LD, Neubauer H. Melioidosis in animals: a review on epizootiology, diagnosis and clinical presentation. J Vet Med B Infect Dis Vet Public Health. 2004;51:305–320. doi: 10.1111/j.1439-0450.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 17.Limmathurotsakul D, Thammasart S, Warrasuth N, Thapanagulsak P, Jatapai A, Pengreugrojanachai V, Anun S, Joraka W, Thongkamkoon P, Saiyen P, Wongratanacheevin S, Day NPJ, Peacock S. Melioidosis in animals, Thailand, 2006–2010. Emerg Infect Dis. 2012;18:325–327. doi: 10.3201/eid1802.111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choy JL, Mayo M, Janmaat A, Currie BJ. Animal melioidosis in Australia. Acta Trop. 2000;74:153–158. doi: 10.1016/s0001-706x(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 19.Sutmoller P, Kraneveld FC, Van Der Schaaf A. Melioidosis (Pseudomalleus) in sheep, goats, and pigs on Aruba (Netherland Antilles) J Am Vet Med Assoc. 1957;130:415–417. [PubMed] [Google Scholar]
- 20.Lloyd JM, Suijdendorp P, Soutar WR. Melioidosis in a dog. Aust Vet J. 1988;65:191–192. doi: 10.1111/j.1751-0813.1988.tb14300.x. [DOI] [PubMed] [Google Scholar]
- 21.McCormick JB, Sexton DJ, McMurray JG, Carey E, Hayes P, Feldman RA. Human-to-human transmission of Pseudomonas pseudomallei. Ann Intern Med. 1975;83:512–513. doi: 10.7326/0003-4819-83-4-512. [DOI] [PubMed] [Google Scholar]
- 22.Galimand M, Dodin A. Le point sur la mélioïdose dans le monde. Bull Soc Pathol Exot. 1982;75:375–383. [PubMed] [Google Scholar]
- 23.Rolim DB, Vilar DC, Sousa AQ, Miralles IS, de Oliveira DC, Harnett G, O'Reilly L, Howard K, Sampson I, Inglis TJJ. Melioidosis, northeastern Brazil. Emerg Infect Dis. 2005;11:1458–1460. doi: 10.3201/eid1109.050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolim DB, Rocha MF, Brilhante RS, Cordeiro RA, Leitão NP, Jr, Inglis TJ, Sidrim JJ. Environmental isolates of Burkholderia pseudomallei in Ceara State, northeastern Brazil. Appl Environ Microbiol. 2009;75:1215–1218. doi: 10.1128/AEM.01953-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomaso H, Pitt TL, Landt O, Al Dahouk S, Scholz HC, Reisinger EC, Sprague LD, Rathmann I, Neubauer H. Rapid presumptive identification of Burkholderia pseudomallei with real-time PCR assays using fluorescent hybridization probes. Mol Cell Probes. 2005;19:9–20. doi: 10.1016/j.mcp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Doker TJ, Sharp TM, Rivera-Garcia B, Perez-Padilla J, Benoit TJ, Ellis EM, Elrod MG, Gee JE, Shieh WJ, Beesley CA, Ryff KR, Traxler RM, Galloway RL, Haberling DL, Waller LA, Shadomy SV, Bower WA, Hoffmaster AR, Walke HT, Blaney DD. Contact investigation of melioidosis cases reveals regional endemicity in Puerto Rico. Clin Infect Dis. 2015;60:243–250. doi: 10.1093/cid/ciu764. [DOI] [PubMed] [Google Scholar]
- 27.Silva SL, Macedo OG, Damasceno CA, Carvalho MA, Cisalpino EO. Bacteriological evaluation of wounds in seriously burned hospitalized patients. Rev Soc Bras Med Trop. 1991;24:163–168. doi: 10.1590/s0037-86821991000300007. [DOI] [PubMed] [Google Scholar]
- 28.Inglis TJ, Rolim DB, Sousa AQ. Melioidosis in the Americas. Am J Trop Med Hyg. 2006;75:947–954. [PubMed] [Google Scholar]
- 29.Glass MB, Steigerwalt AG, Jordan JG, Wilkins PP, Gee JE. Burkholderia oklahomensis sp. nov., a Burkholderia pseudomallei-like species formerly known as the Oklahoma strain of Pseudomonas pseudomallei. Int J Syst Evol Microbiol. 2006;56:2171–2176. doi: 10.1099/ijs.0.63991-0. [DOI] [PubMed] [Google Scholar]
- 30.Dance DA. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond HS, Pastore R. Septic arthritis due to Pseudomonas pseudomallei. Arthritis Rheum. 1967;10:459–466. doi: 10.1002/art.1780100508. [DOI] [PubMed] [Google Scholar]
- 32.Central Intelligence Agency The World Factbook. 2014. https://www.cia.gov/library/publications/the-world-factbook/index.html Available at. Accessed December 3, 2015.
- 33.Zehnder AM, Hawkins MG, Koski MA, Lifland B, Byrne BA, Swanson AA, Rood MP, Gee JE, Elrod MG, Beesley CA, Blaney DD, Ventura J, Hoffmaster AR, Beeler ES. Burkholderia pseudomallei isolates in 2 pet iguanas, California, USA. Emerg Infect Dis. 2014;20:304–306. doi: 10.3201/eid2002.131314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osteraas GR, Hardman JM, Bass JW, Wilson C. Neonatal melioidosis. Am J Dis Child. 1971;122:446–448. doi: 10.1001/archpedi.1971.02110050116019. [DOI] [PubMed] [Google Scholar]
- 35.Garry MW, Koch ML. Chronic melioidosis: bacteriologic and clinical correlation in diagnosis. J Lab Clin Med. 1951;38:374–383. [PubMed] [Google Scholar]
- 36.Stewart T, Engelthaler DM, Blaney DD, Tuanyok A, Wangsness E, Smith TL, Pearson T, Komatsu KK, Keim P, Currie BJ, Levy C, Sunenshine R. Epidemiology and investigation of melioidosis, southern Arizona. Emerg Infect Dis. 2011;17:1286–1288. doi: 10.3201/eid1707.100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doker TJ, Quinn CL, Salehi ED, Sherwood JJ, Benoit TJ, Glass Elrod M, Gee JE, Shadomy SV, Bower WA, Hoffmaster AR, Walke HT, Blaney DD, DiOrio MS, Melioidosis Investigation Team Fatal Burkholderia pseudomallei infection initially reported by Bacillus species, Ohio. Am J Trop Med Hyg. 2014;91:743–746. doi: 10.4269/ajtmh.14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beamer PR, Varney PL. Melioidosis; report of second case from the Western Hemisphere, with bacteriologic studies on both cases. Am J Pathol. 1948;24:717. [PubMed] [Google Scholar]
- 39.Olive C, Loetitia G, Desbois N, Roche B, Jouannelle J, Dodin A. Forme septicopyohemique de melioidose humanie: un premier cas aux Antilles Francaises. Presse Med. 1995;24:1270. [PubMed] [Google Scholar]
- 40.Gétaz L, Abbas M, Loutan L, Schrenzel J, Iten A, Simon F, Decosterd A, Studer R, Sudre P, Michel Y, Merlani P, Emonet S. Fatal acute melioidosis in a tourist returning from Martinique Island, November 2010. Euro Surveill. 2011;16:pii 19758. [PubMed] [Google Scholar]
- 41.Brilhante RS, Bandeira TJ, Cordeiro RA, Grangeiro TB, Lima RA, Ribeiro JF, Castelo-Branco DS, Rodrigues JL, Coelho IC, Magalhães FG, Rocha MF, Sidrim JJ. Clinical-epidemiological features of 13 cases of melioidosis in Brazil. J Clin Microbiol. 2012;50:3349–3352. doi: 10.1128/JCM.01577-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miralles IS, Maciel MC, Angelo MR, Gondini MM, Frota LH, dos Reis CM, Hofer E. Burkholderia pseudomallei: a case report of a human infection in Ceará, Brazil. Rev Inst Med Trop Sao Paulo. 2004;46:51–54. doi: 10.1590/s0036-46652004000100011. [DOI] [PubMed] [Google Scholar]
- 43.Liguori AP, Warrington SD, Ginther JL, Pearson T, Bowers J, Glass MB, Mayo M, Wuthiekanun V, Engelthaler D, Peacock SJ, Currie BJ, Wagner DM, Keim P, Tuanyok A. Diversity of 16S-23S rDNA internal transcribed spacer (ITS) reveals phylogenetic relationships in Burkholderia pseudomallei and its near-neighbors. PLoS One. 2011;6:e29323. doi: 10.1371/journal.pone.0029323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gee JE, Allender CJ, Tuanyok A, Elrod MG, Hoffmaster AR. Burkholderia pseudomallei type G in Western Hemisphere. Emerg Infect Dis. 2014;20:682–684. doi: 10.3201/eid2004.130960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benoit TJ, Blaney DD, Gee JE, Elrod MG, Hoffmaster AR, Doker TJ, Bower WA, Walke HT. Melioidosis cases and selected reports of occupational exposures to Burkholderia pseudomallei—United States, 2008–2013. MMWR. 2015;64:1–9. [PubMed] [Google Scholar]
- 46.Hoffmaster AR, AuCoin D, Baccam P, Baggett HC, Baird R, Bhengsri S, Blaney DD, Brett PJ, Brooks TJ, Brown KA, Chantratita N, Cheng AC, Dance DA, Decuypere S, Defenbaugh D, Gee JE, Houghton R, Jorakate P, Lertmemongkolchai G, Limmathurotsakul D, Merlin TL, Mukhopadhyay C, Norton R, Peacock SJ, Rolim DB, Simpson AJ, Steinmetz I, Stoddard RA, Stokes MM, Sue D, Tuanyok A, Whistler T, Wuthiekanun V, Walke HT. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis. 2015;21:1–9. doi: 10.3201/eid2102.141045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.