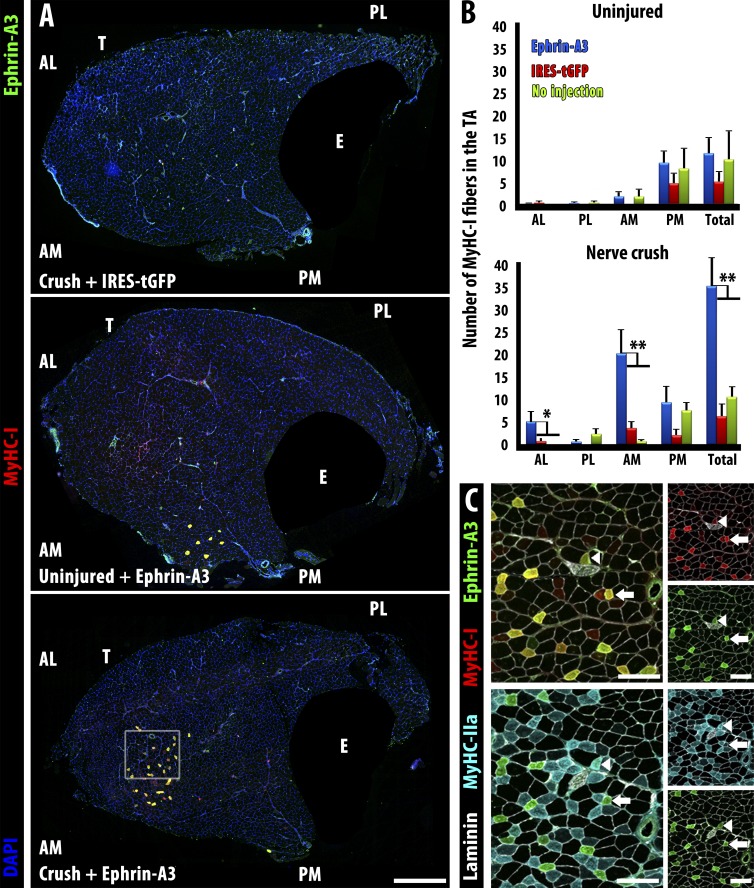
Figure 5.
Misexpression of ephrin-A3 in the TA by electroporation followed by sciatic nerve crush imposes a fast to slow fiber type switch by 28 d postcrush. (A) Neither electroporation with a control plasmid (IRES-tGFP) followed by nerve crush (top) nor electroporation with ephrin-A3 plasmid without nerve crush (middle) significantly changed the number of slow myofibers in wild-type TA muscles compared with sham-operated controls. However, electroporation with ephrin-A3 plasmid followed by challenge with nerve crush led to significant conversion of fast myofibers to slow (MyHC-I+ve) at the injection site (bottom). EDL (E) is masked in all panels. Bar, 500 µm. (B) Quantification of slow myofibers in different quadrants of the TA in the experiments described above: AL, anterior lateral; AM, anterior medial; PL, posterior lateral; PM, posterior medial. Plasmid DNA was injected into the anterior aspect of the TA followed by electroporation. Only electroporation with ephrin-A3 in conjunction with nerve crush (blue bars, bottom) significantly increases MyHC-I+ve myofibers. Error bars = SEM. *, P < 0.05; **, P < 0.01. (C) Serial sections corresponding to inset box in A showing coexpression of ephrin-A3 (green) with either MyHC-I (red, top) or MyHC-IIa (cyan, bottom). Laminin is shown in white. All ephrin-A3+ve myofibers express MyHC-I (i.e., arrows); a few (arrowheads) express both MyHC-I and MyHC-IIa. Bars, 100 µm. T, TA; E, EDL.