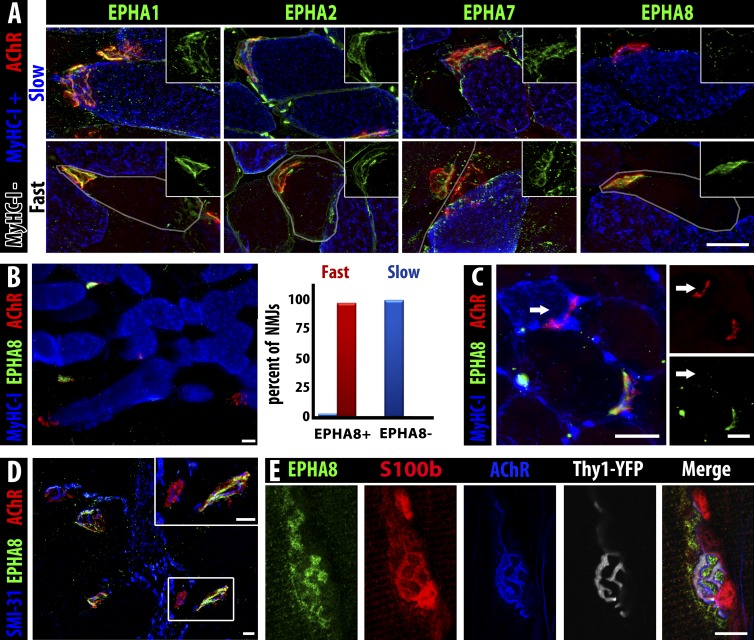
Figure 7.
EphA8 is expressed by terminal Schwann cells associated with fast myofiber NMJs. (A) EphA1, EphA2, and EphA7 (green) are present on NMJs (α-bungarotoxin, red) of both fast myofibers (gray outline) and slow myofibers (MyHC-I, blue), but EphA8 (green, right) is only detectable at NMJs of fast myofibers. Insets show green staining for the indicated Eph at the same magnification. Bar, 25 µm. (B) Section of soleus muscle (MyHC-I+ve myofibers, blue) showing expression of EphA8 (green) at fast but not slow myofiber NMJs (α-bungarotoxin, red). Bar, 25 µm. 100% of fast myofiber NMJs were positive for EphA8 (red bar), whereas almost all (97%) slow myofiber NMJs lacked EphA8 staining (blue bar); the very small fraction (<3%) of EphA8+ve NMJs associated with MyHC-I+ve myofibers may represent hybrid myofibers. n = 134 NMJs. (C) A fast TA myofiber converted to slow by ephrin-A3 misexpression/nerve crush does not have EphA8 at its NMJ: arrow indicates the NMJ of the same slow myofiber marked with an arrow in Fig. 5 C (all 13 converted myofibers scored were EphA8 negative). Bars, 25 µm. (D) EphA8 (green) expression is localized at the NMJ (α-bungarotoxin, red) and not associated with the motor axon (SMI-31, blue). Bars, 25 µm. α-Bungarotoxin gamma = 1.45. (E) Confocal analysis of staining for presynaptic neuron (Thy1-YFP, gray), postsynaptic myofiber (acetylcholine receptor, blue), and Schwann cell cytoplasm (S100b, red) consistent with expression of EphA8 (green) by terminal Schwann cells. Note that because S100b is cytoplasmic and EphA8 is at the cell surface, overlap of staining is not necessarily expected in these confocal sections. Bar, 10 µm.