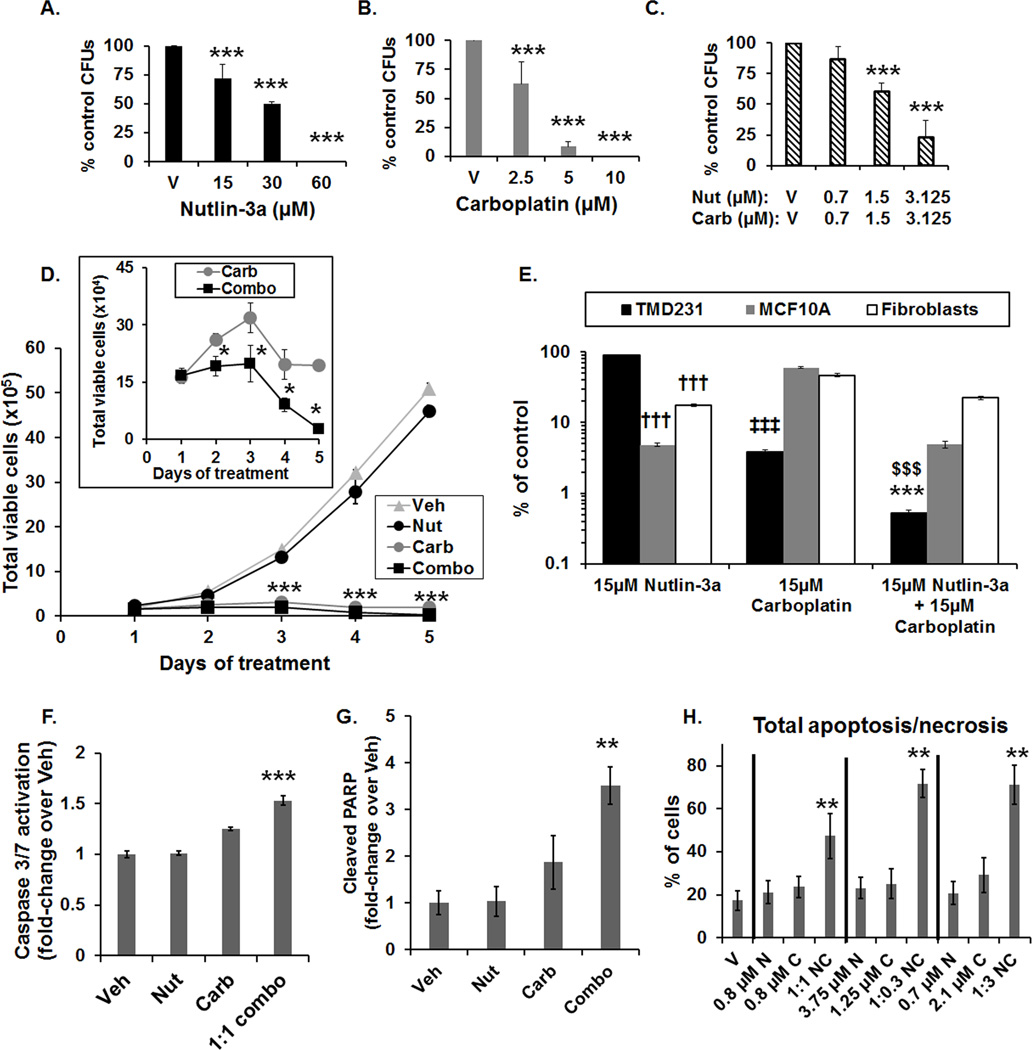
Figure 2. Potentiation of carboplatin-mediated DNA damage by Nutlin-3a increases cell death and differentially affects normal cells.
(A–C) Dose-dependent reduction in colony forming units (CFUs) by Nutlin-3a (A), carboplatin (B), and in the 1:1 combination (C) (***p<0.001 vs V (vehicle), Holm-Sidak post hoc test, n=3). (D) Time-course of the number of viable TMD231 cells following treatment with vehicle (Veh), 15 µM Nutlin-3a (Nut), 15 µM carboplatin (Carb) or 1:1 combination (Combo) (***p<0.001 combination vs Vehicle, Holm-Sidak post hoc test, n=3). Inset: Number of viable cells following carboplatin and combination treatment (*p<0.05 vs carboplatin alone, Student’s t-test, n=3). Vertical lines indicate ±1 SD and are absent when less than the size of the point. (E) Effects of 15 µM of Nutlin-3a, carboplatin, or 1:1 combination in TMD231, MCF10A mammary epithelial, and human fibroblast cells on the number of viable cells, expressed as % of vehicle control, following 5-days of treatment. The y-axis was plotted on a log scale to better illustrate the comparisons. (††† p<0.001 vs TMD231 treated with 15 µM Nutlin-3a, ‡‡‡ p<0.001 vs MCF10A and fibroblasts treated with 15 µM carboplatin, $$$ p<0.001 vs MCF10A and fibroblasts treated with combination, ***p<0.001 vs TMD231 treated with 15 µM Nutlin-3a and carboplatin alone, Holm-Sidak post hoc test, n=3). (F) Activated Caspase-3/7 fold-induction in TMD231 cells treated with Vehicle (Veh), 15 µM Nutlin-3a (Nut), 15 µM carboplatin (Carb), or the combination (1:1 combo) for 3 days (***p<0.001 vs Veh, Nut, and Carb, Holm-Sidak post hoc test, n=3). (G) Cleaved PARP fold-induction in TMD231 cells treated with Vehicle (Veh), 15 µM Nutlin-3a (Nut), 15 µM carboplatin (Carb), or the combination (1:1 combo) for 3 days (***p<0.01 vs Veh, Student’s t-test, n=3, ±SEM). (H) Total apoptosis/necrosis in TMD231 cells treated with Nutlin-3a (N), carboplatin (C), or the combination (NC) at the indicated doses and dose-ratios (**p<0.01 vs Veh, N, and C, Holm-Sidak post hoc test, n=3).