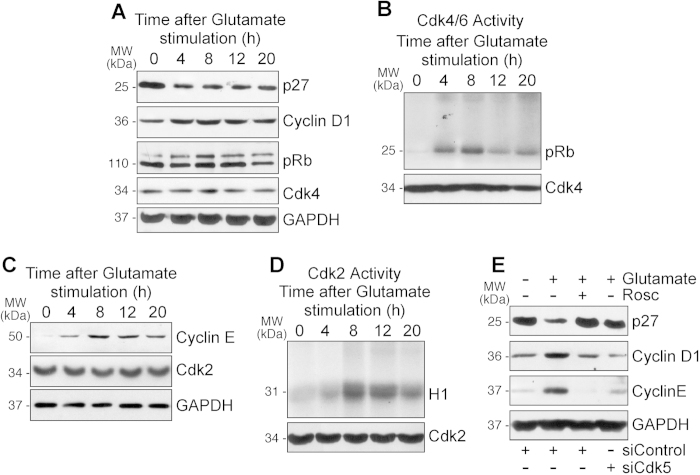
Figure 3. Glutamatergic stimulation causes p27 depletion and activation of the cell cycle machinery in postmitotic neurons through a Cdk5-mediated mechanism.
Rat cortical neurons were treated with glutamate (100 μM, 5 min) and were further incubated in culture medium for 4–20 h. Levels of (A) p27, cyclin D1, retinoblastoma protein (pRb), and Cdk4 and (C) levels of cyclin E and Cdk2 were detected by Western blotting. GAPDH protein levels were used as loading control. (B,D) Neuronal extracts were immunoprecipitated with anti-Cdk4 (B) and anti-Cdk2 (D) antibodies. Kinase activities were assayed as the ability to phosphorylate pRb (1 mg/ml), for Cdk4, and histone H1 (1 mg/ml), for Cdk2, in vitro. Samples were subjected to SDS-polyacrylamide gel (12%) electrophoresis and transferred proteins were visualized by autoradiography or blotted with anti-Cdk4 and anti-Cdk2. (E) Rat cortical neurons were transfected with siRNA against Cdk5 (100 nM siCdk5) for 3 days. Neurons were then treated with glutamate (100 μM, 5 min) and were further incubated in culture medium, supplemented with 10 μM roscovitine (Rosc), for 8 hours. Inhibition of Cdk5 activation by both roscovitine treatment and siCdk5 transfection prevented changes in cells cycle proteins (p27, cyclin D1 and cyclin E) induced by excitotoxicity. In all cases, a representative western blot is shown out of three. The relative protein abundance is shown in Supplementary Fig. 2.