Figure 1. The vicinal dichloride motif in natural products.
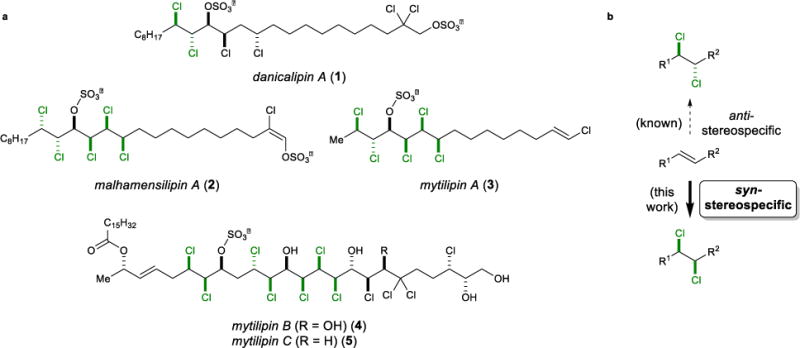
a, Selected chlorosulfolipids (with vicinal dichloride motifs highlighted). b, Most dichlorinations of alkenes (and all of those that are catalytic) exhibit anti-stereospecificity, and no generally applicable, direct syn-stereospecific alkene dichlorination has been reported. Such a method would complement current chlorination strategies employed in the synthesis of polychlorinated natural products such as the chlorosulfolipids.
