Table 1.
Reaction Development with Cyclohexene
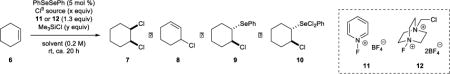
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | Cl− source (equiv) | oxidant | Me3SiCl equiv | solvent | NMR yield (%) a
|
||||
| 6 | 7 | 8 | 9 | 10 | |||||
| 1 | n-Bu4NCl (3.0) | 11 | 0.0 | MeCN-d3 | 50 | 19 | 3 | 9 | 0 |
| 2 | n-Bu4NCl (3.0) | 11 | 1.0 | MeCN-d3 | 12 | 61 | 10 | 10 | 0 |
| 3 | n-Bu4NCl (3.0) | 11 | 2.0 | MeCN-d3 | 0 | 81 | 10 | 0 | 0 |
| 4 | n-Bu4NCl (3.0) | 11 | 3.0 | MeCN-d3 | 0 | 81 | 8 | 0 | 0 |
| 5 | n-Bu4NCl (2.5) | 11 | 2.0 | MeCN-d3 | 0 | 74 | 10 | 0 | 0 |
| 6b | n-Bu4NCl (0.0) | 11 | 2.0 | MeCN-d3 | 54 | 0 | 2 | 0 | 8 |
| 7 | n-Bu4NCl (3.0) | 11 | 2.0 | CD2Cl2 | 0 | 73 | 12 | 4 | 0 |
| 8 | n-Bu4NCl (3.0) | 11 | 2.0 | THF-d8 | 55 | 17 | 2 | 0 | 0 |
| 9 | n-Bu4NCl (3.0) | 12 | 2.0 | MeCN-d3 | 0 | 71 | 10 | 0 | 0 |
| 10 | BnEt3NCl (3.0) | 11 | 2.0 | MeCN-d3 | 0 | 83 | 10 | 0 | 0 |
Measured by 1H NMR spectroscopy with 1,1,2,2-tetrachloroethane (1.0 equiv) as an internal standard.
11% of an unidentified species was also observed by 1H NMR spectroscopy.
