Table 3.
Reaction Generality.
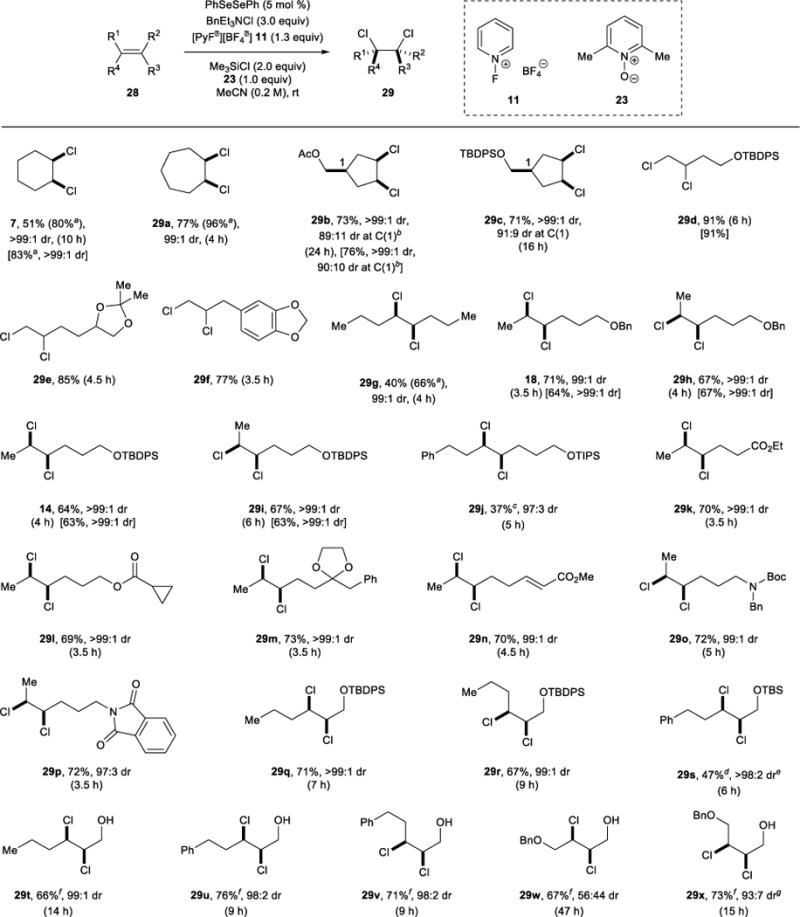
|
For comparison, yields and diastereoselectivities for selected reactions conducted for 18 h without additive 23 are given in square brackets. Each diastereomeric ratio (dr) was determined on the crude mixture after passing through a short plug of silica gel.
Yield was determined by 1H NMR spectroscopy with 1,1,2,2-tetrachloroethane (1.0 equiv) as an internal standard.
91:9 dr at C(1) after isolation.
24% of the free alcohol syn-dichloride was also isolated, and tentatively assigned cyclized products were also observed in the crude product mixture.
21% of the free alcohol syn-dichloride was also isolated.
The dr corresponds to the sum of the dichlorides.
Additive 23 was omitted.
97:3 dr after isolation.
