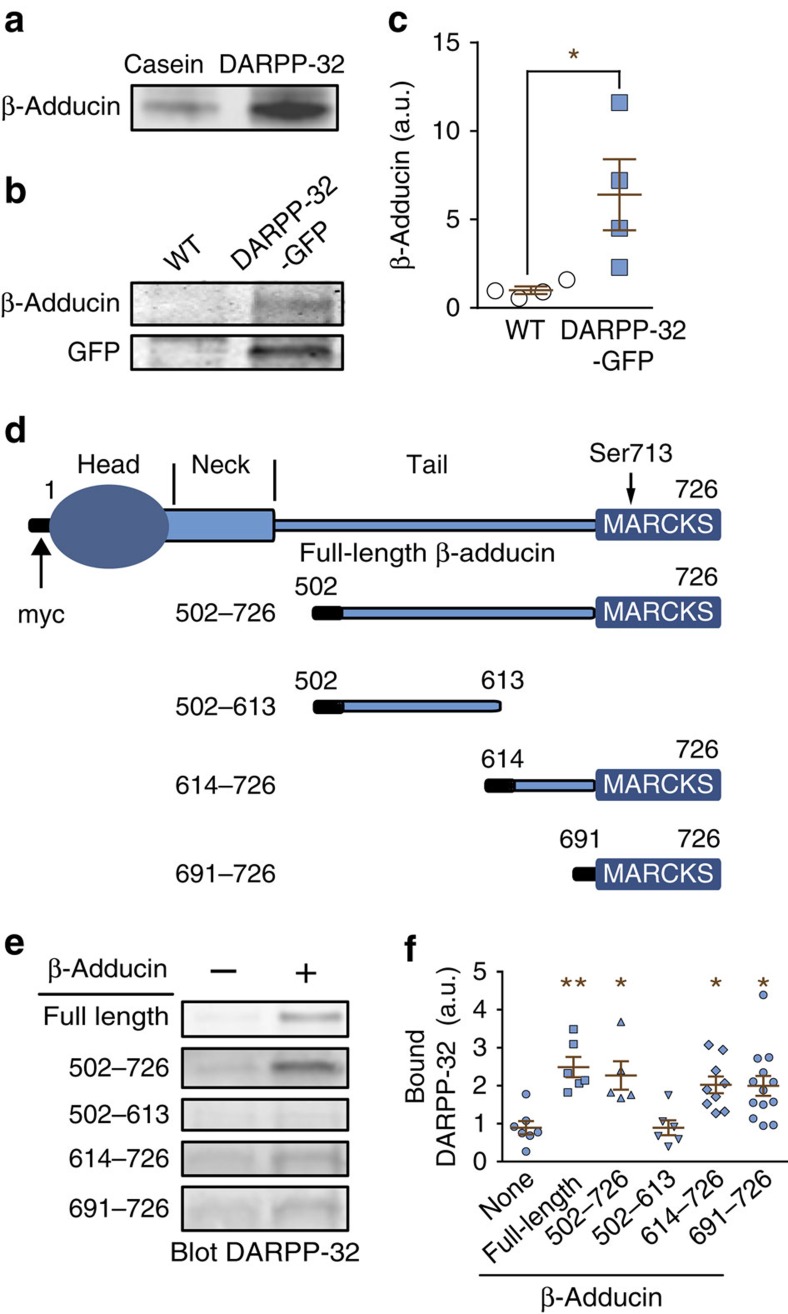
Figure 1. DARPP-32 interacts with the MARCKS domain of β-adducin.
(a) Rat striatal lysates were incubated with Sepharose coupled to purified recombinant DARPP-32 or to casein as control. After washing, proteins were eluted with 150 mM NaCl, analysed by SDS–polyacrylamide gel electrophoresis and immunoblotted for β-adducin. (b) Striatal lysates from DARPP-32-GFP transgenic mice and WT littermates were incubated with Sepharose-bound GFP antibody. Immune precipitates were analysed by immunoblotting for β-adducin (upper panel) or GFP (lower panel). (c) The amounts of β-adducin co-immunoprecipitated with GFP from WT or DARPP-32-GFP transgenic mice as in c were quantified. Independent data points are plotted and means±s.e.m. are shown, n=4 per group, Student's t-test, t6=2.68, *P<0.05. (d) Domain organization of β-adducin and its fragments used in the current study (all fused to an N-terminal 13-residue myc peptide, black). MARCKS, myristoylated alanine-rich C-kinase substrate homology domain. The position of the phosphorylated Ser713 in the MARCKS domain is indicated. (e) COS-7 cells were transfected with empty myc vector (−) or myc-tagged full-length β-adducin or its fragments (+). Myc-tagged proteins were immunoprecipitated with agarose-coupled myc antibody. Beads were then incubated with lysates of DARPP-32-GFP-transfected cells and bound DARPP-32 detected by immunoblotting. (f) Quantification of DARPP-32-GFP association with β-adducin fragments. The background level is indicated by the amount of DARPP-32 precipitated with beads coated with lysates of myc vector-transfected COS-7 cells (−, dotted line). Independent data points are plotted and means±s.e.m. are shown; n=5–13 per group from >3 experiments; one-way ANOVA: F(5,40)=5.82, P<0.001. Tukey‘s test versus no β-adducin (-): *P<0.05; **P<0.01. Independent data points are plotted and means±s.e.m. are shown.