Abstract
Background
Hip flexor tightness is theorized to alter antagonist muscle function through reciprocal inhibition and synergistic dominance mechanisms. Synergistic dominance may result in altered movement patterns and increased risk of lower extremity injury.
Hypothesis/Purpose
To compare hip extensor muscle activation, internal hip and knee extension moments during double‐leg squatting, and gluteus maximus strength in those with and without clinically restricted hip flexor muscle length.
Design
Causal‐comparative cross‐sectional laboratory study.
Method
Using a modified Thomas Test, female soccer athletes were assigned to a restricted (>0 ° of sagittal plane hip motion above the horizontal; n=20, age=19.9 ± 1 years, ht=167.1 ± 6.4 cm, mass=64.7 ± 8.2kg) or normal (>15 ° of sagittal plane hip motion below horizontal; n=20, age=19.4 ± 1 years, ht=167.2 ± 5.5 cm, mass=61.2 ± 8.6 kg) hip flexor muscle length group. Surface electromyographic (sEMG) activity of the gluteus maximus and biceps femoris, and net internal hip and knee extension moments were measured between groups during a double‐leg squat. Isometric gluteus maximus strength was assessed using handheld dynamometry.
Results
Individuals with restricted hip flexor muscle length demonstrated less gluteus maximus activation (p=0.008) and a lower gluteus maximus : biceps femoris co‐activation ratio (p=0.004). There were no significant differences (p>0.05) in hip or knee extension moments, isometric gluteus maximus strength, or biceps femoris activation between groups.
Conclusions
Female soccer athletes with hip flexor muscle tightness exhibit less gluteus maximus activation and lower gluteus maximus : biceps femoris co‐activation while producing similar net hip and knee extension moments. Thus, individuals with hip flexor muscle tightness appear to utilize different neuromuscular strategies to control lower extremity motion.
Level of Evidence
3
Keywords: ACL Injury, Electromyography, Hamstring Injury, Musculoskeletal Injury, Neuromuscular Control
INTRODUCTION
Lower extremity injuries represent a significant burden in sports and physical activity, contributing to a majority of time loss from participation and disability.1‐7 Furthermore, lower extremity injuries contribute to decreased athletic performance and overall team success.8 However, a majority of lower extremity and lumbo‐pelvic‐hip complex injuries, including hamstring injury,7 groin injury,9 ankle sprains,10 anterior cruciate ligament rupture,11‐14 and low back pain,15,16 have been shown to be preventable and are attributable to modifiable biomechanical risk factors.17 Restricted hip flexor muscle length or “tightness” assessed via hip extension range of motion (ROM)18 has been identified as a risk factor for various lower extremity musculoskeletal injuries,15,16,19‐23 and thus should be examined further as a modifiable factor linked to sport‐related injury.
Furthermore, restricted hip flexor muscle length is theorized to decrease neural drive to the hip extensor musculature. Specifically, reciprocal inhibition of the gluteus maximus, secondary to “overactivity” of the hip flexor muscle group has been implicated to occur and lead to lower extremity injury.24‐27 Reciprocal inhibition is theorized to lead to an increased reliance on the secondary hip extensor muscles, such as the hamstrings and hip adductors to produce hip extension torque,28 clinically referred to as “synergistic dominance”.29 Dependency on secondary hip extensors may provoke greater tissue stress in the hamstring and hip adductor musculature, thus resulting in a higher risk of soft tissue injury.30‐32 However there is a dearth of literature that validates clinical theory of restricted hip flexor muscle length as an underlying factor inciting altered lower extremity neuromuscular control.
Therefore, the purpose of this study was to compare lower extremity strength, muscle activation and biomechanics between individuals with and without limited hip flexor muscle length. The primary hypothesis of this study was that individuals with restricted hip flexor length would exhibit less hip extension strength, greater internal knee extension moment, and lesser internal hip extension moment compared to individuals with normal hip flexor length during the descent phase of a double‐leg squat (DLS). The secondary hypothesis of this investigation was that individuals with restricted hip flexor length would also display depressed gluteus maximus activation and elevated biceps femoris activation compared to those with normal hip flexor length during the descent phase of a DLS.
METHODS
Participants
The investigators conducted a causal‐comparative study involving forty female soccer athletes who demonstrated “restricted” (n=20) or “normal” (n=20) hip flexor length. All participants, regardless of group assignment, played soccer at the NCAA Division I varsity or the highest the competitive intercollege club level for one hour or more at least twice a week, had no history of lower extremity, spine, abdominal, vestibular, or mild traumatic brain injury in the last three months that limited them from sport of physical activity participation for greater than three consecutive days. A priori power analyses revealed that 20 participants per group would result in an estimated power of 0.80 to observe significant differences of at least 20% in muscle activity, biomechanics, and strength between the normal and restricted groups.33‐35
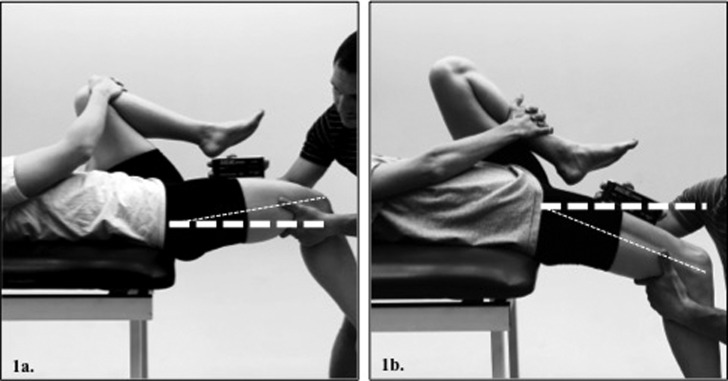
Hip extension ROM of the dominant limb was assessed by the lead investigator (MM), a certified athletic trainer, using the modified Thomas test assessed using a digital inclinometer (Model# 12‐1057, Fabrication Enterprises Inc. ‐ Baseline Evaluation Instruments, White Plains, New York) aligned parallel to a line connecting the anterior superior iliac spine and the superior pole of the patella (Figure 1).18,36‐38 Pilot testing revealed excellent intra‐rater reliability (ICC(3,k)=0.99) with a low standard error of measure (SEM=0.85 º). The dominant leg was defined as the leg the participant would use to kick as soccer the ball furthest. Previous research has demonstrated this method of hip extension ROM assessment to have good inter‐rater reliability (ICC(2,1)=0.89–0.92 & SEM=3‐2.1 º).36‐38
Figure 1.
Modified Thomas Test assessment of hip extension ROM to assess hip flexor muscle length; (1a.) – restricted > 0 º above the horizontal, (1b.) – normal > 15 º below the horizontal.
Inclinometer values greater than 0 ° (+) indicate that the thigh was positioned above the horizontal and relatively flexed (Figure 1a).18 Inclinometer values below 0 ° (‐) indicate that the thigh was below the horizontal and relatively extended (Figure 1b).18 Inclusion criteria for the normal group was defined as hip extension ROM >15 ° below the horizontal. Inclusion criteria for the restricted group was defined as hip extension ROM >0 ° above the horizontal.
Experimental Procedures
Before participation, all study procedures were explained to each subject, and informed consent was obtained as approved by the Institutional Review Board at The University of North Carolina at Chapel Hill. Participants’ height (cm) and mass (kg) were recorded using a digital scale and stadiometer. Prior to testing, participants completed a warm‐up on a stationary cycle ergometer at a self‐selected pace for five minutes at a rate of perceived exertion of 5/10.
Maximal Volitional Isometric Contraction Assessment
A surface electromyography (sEMG) system (Delsys Bangloni 8, Inc., Boston, Massachusetts,): inter‐electrode distance =10 mm; amplification factor =1,000 (20–450 Hz); CMMR @60 Hz > 80 dB; input impedance > 1015//0.2 W//pF) was used to record activity as sampled at 1,000 Hz from the gluteus maximus and biceps femoris of the dominant limb during maximal volitional contractions (MVIC). Electrodes for the biceps femoris were placed approximately 1/3rd the distance between the ischial tuberosity and lateral popliteal crease, while those for the gluteus maximus were placed approximately 1/3rd the distance between the second sacral vertebrae and the greater trochanter.39 A reference electrode was placed at the tibial tuberosity on the dominant leg.
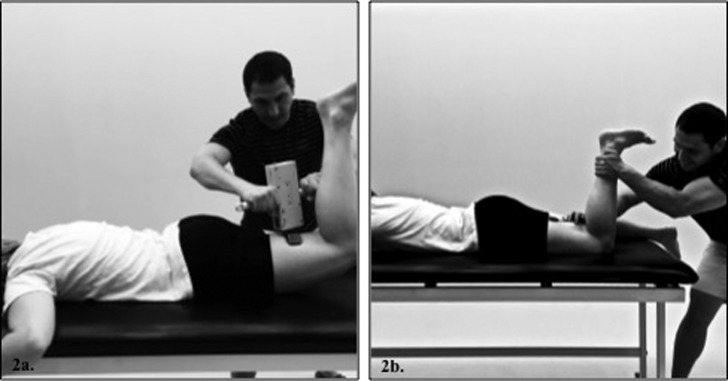
Dominant limb peak isometric force and sEMG measures were concurrently collected for the gluteus maximus with the knee flexed 90 º (Figure 2a) by the lead investigator (MM). Hip extension strength data were obtained via a handheld digital dynamometer (Lafayette Manual Muscle Tester, Model #01163, Lafayette, Indiana). Isometric hip extension strength pilot testing revealed excellent intra‐rater reliability (ICC(3,k)=0.98) and a low standard error of the measure (SEM=0.48 N). Isometric force (N) and sEMG activity were collected for five seconds for each of three trials. For the biceps femoris, participants maximally flexed their knee against the investigator‐applied force to the posterior shank at the level of the medial and lateral malleoli (Figure 2b). sEMG data were collected for five seconds for each of three trials.
Figure 2.
(2a.) – Gluteus maximus MVIC & strength assessment, (2b.) – Biceps femoris MVIC assessment.
Biomechanical Assessment Preparation & Assessment
A TrackStar (Ascension Technologies Inc. Burlington, Vermont, USA) electromagnetic motion analysis system controlled by The Motion Monitor v8.0 software (Innovative Sports Training Inc. Chicago, Illinois, USA) was used to sample hip and knee kinematic data at 100 Hz during the DLS. Electromagnetic sensors were secured to the participant's dominant limb shank and thigh, and the apex of the sacrum. Medial femoral epicondyle, lateral femoral epicondyle, medial malleolus, lateral malleolus, left anterior superior iliac spine, right anterior superior iliac spine bony landmarks were digitized using a 15 cm stylus attached to an electromagnetic sensor. The knee and ankle joint centers were defined as the centroids between the medial and lateral femoral condyles and medial and lateral malleoli. The Bell method was used to approximate the hip joint center.40 A non‐conductive force plate (Bertec 4060‐NC, Columbus, Ohio, USA) sampled center of pressure and ground reaction force data at 1,000 Hz.
A right‐handed global coordinate system was defined for all segments (+x‐axis= anterior direction, + y‐axis= leftward direction and +z‐axis= superior direction). Sagittal plane joint motion was defined as the motion of the distal segment relative to the proximal segment using a Cardan angle rotation sequence with the first rotation about the y‐axis of the joint.41
Muscle activation, kinematic, and kinetic data were collected during the descent phase of the DLS to simulate hip and knee flexion deceleration neuromuscular control during sport participation. Subjects performed the five DLS repetitions barefoot with their feet shoulder width apart, toes pointed straight ahead, and arms extended over‐head. Squat velocity was controlled via a metronome (60 beats per minute).32 Participants achieved at least 60 º of knee flexion confirmed with a goniometer and motion capture knee kinematics.32 Participants were instructed to descend for two beats, ascend for two beats, and then pause for one beat between squats and then repeat. Prior to assessment, participants were required to perform between five and seven consecutive practice trials of squatting at the appropriate depth and cadence for familiarization.
Data Reduction
Peak isometric force (N) for each gluteus maximus MVIC trial was averaged across the three testing trials and normalized to the participant's body weight (×BW).
Raw sEMG data were exported into a custom MatLab v2012a program (MathWorks, Natick, Massachusetts, USA.) and then passively demeaned, band‐pass (10‐350 Hz) and notch filtered (59.5‐60.5 Hz ‐ 4th order Butterworth digital filter), and smoothed using a 25 ms root mean squared sliding window. sEMG data were normalized as a percentage of MVIC (%MVIC).
Mean sEMG amplitudes were calculated during the descending phase of each trial of the DLS, defined as the period from initiation of knee flexion until peak knee flexion. Muscle co‐activation ratios were calculated during the descending phase for gluteus maximus and biceps femoris muscle activation by dividing the mean gluteus maximus activity by the mean biceps femoris activity (gluteus maximus : biceps femoris). A ratio of 1.0 indicates balanced muscular activation; ratios less than 1.0 indicate greater activation of biceps femoris relative to the gluteus maximus.
All kinematic data were low‐pass filtered at 10 Hz (4th order low‐pass Butterworth digital filter). Kinematic and kinetic data were combined via an inverse dynamics solution to yield net internal hip and knee extension moments (Nm). Peak moments were identified during the descending phase of each squat trial, normalized to the product of the body weight and height (BW×Ht), and averaged across trials. Internal hip and knee extension moment data are reported as positive values for ease of interpretation.
STATISTICAL ANALYSES
Separate independent samples t‐tests were performed to compare hip extension ROM, gluteus maximus strength, gluteus maximus activation, biceps femoris activation, gluteus maximums : biceps femoris co‐activation ratio, and peak hip and knee extension moments between the restricted and normal groups (α=0.05).
Statistical outliers were defined as variables with values more than three standard deviations from the group means. Examination of sEMG data identified outliers in biceps femoris (n=8) and gluteus maximus (n=5) sEMG data. The total number of subjects utilized in analyses of sEMG data is presented in Table 1. No statistical outliers were identified for internal moment or strength data.
Table 1.
Muscle activations (%MVIC) during the descent phase of a double‐leg squat
| Restricted | Normal | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | 95% CI | n | Mean (SD) | 95% CI | p | Cohen's d | |
| Gluteus Maximus* | 17 | 7.5 (3.8) | [5.7, 9.3] | 18 | 12.4 (6.3) | [9.5, 15.3] | 0.005 | 0.94 |
| Biceps Femoris | 18 | 8.2 (3) | [6.7, 9.7] | 14 | 7.1 (3.8) | [5.3, 8.9] | 0.384 | 0.32 |
| Co‐Activation Ratio†* | 17 | 87.8 (34.1) | [69.9, 105.7] | 14 | 228.4 (168.3) | [148.4, 308.4] | 0.004 | 1.11 |
Significant difference (p ≤ 0.05) between the restricted and control group.
Co‐Activation ratio is calculated as %MVIC gluteus maximus : %MVIC biceps femoris.
RESULTS
Inclusion Criteria Verification
There was a significant difference in hip extension ROM p < 0.001, d =2.18) between the restricted (12.85 ° ± 5.05) and normal (‐19.52 ° ± 9.19 °) groups, with no significant group differences in age, height, or mass (Restricted: age=19.9 ± 1 years, ht=167.1 ± 6.4 cm, mass=64.7 ± 8.2kg, Normal: age=19.4 ± 1 years, ht=167.2 ± 5.5 cm, mass=61.2 ± 8.6 kg, p > 0.05).
Muscle Activation
Descriptive statistics, p‐values, and effect sizes are presented for all DLS muscle activation data in Table 1. The restricted group demonstrated significantly less average gluteus maximus activation compared to the normal group during the descent phase of the DLS (60% less relative activation). The restricted group also displayed a significantly lower gluteus maximus : biceps femoris co‐activation ratio than the normal group, indicating 2.6 times more biceps femoris activation relative to gluteus maximus activity in the restricted compared to the control group. However, biceps femoris EMG amplitude normalized to MVIC did not differ between groups
Muscle Strength & Joint Moments
There were no significant differences between the restricted and normal groups in hip extension strength or internal hip and knee extension moments during the DLS. Descriptive statistics, p‐values, and effect sizes for these data are presented in Table 2.
Table 2.
Net normalized internal hip & knee extension moments (Nm/NBW/mHT) during the descent phase of a double‐leg squat, & isometric gluteus maximus strength (N/NBW).
| Restricted | Normal | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | p | Cohen's d | |
| Hip Extension Moment | 0.091 (0.021) | [0.082, 0.1] | 0.096 (0.03) | [0.083, 0.109] | 0.580 | 0.19 |
| Knee Extension Moment | 0.072 (0.038) | [0.055, 0.089] | 0.073 (0.073) | [0.041, 0.105] | 0.360 | 0.02 |
| Gluteus Maximus Strength | 0.39 (0.21) | [0.298, 0.482] | 0.4 (0.14) | [0.34, 0.46] | 0.730 | 0.06 |
DISCUSSION
This is the first study to compare gluteal muscle strength, primary hip extensor muscle activation, and lower extremity biomechanics between individuals with restricted and normal hip flexor muscle length during a controlled functional movement. The current findings revealed that muscle activation amplitude of the gluteus maximus was significantly less in the restricted group compared to the normal group, resulting in a significantly lower gluteus maximus : biceps femoris co‐activation ratio. However, there was no difference in biceps femoris muscle activation amplitude, gluteus maximus strength, or internal hip and knee extension moments between groups. These findings support the hypothesis that gluteus maximus activation is affected by hip flexor muscle length. Furthermore, these findings implicate that individuals with restricted hip flexor length may use less muscle activation of the gluteus maximus and greater relative activation of the hamstrings to achieve the same net hip extension moment profile as those with normal hip flexor muscle length.
The restricted group had a relative difference of 60% less gluteus maximus activation compared to the normal group during the descending phase of the squat. The gluteus maximus is the primary muscle to eccentrically control hip flexion motion by generating an internal hip extension moment.28,42 Given the function of the gluteus maximus and the relatively large decrease in activity of this muscle, the investigators expected to observe smaller internal hip extension and a compensatory knee extension strategy43 resulting in a greater internal knee extension moment in the restricted group. However, there was no difference in hip or knee extension moments between groups. Thus, the groups achieved the same net hip extension moment during the descent phase of the squat, but through different muscle activation strategies.
While not significantly different, the restricted group exhibited 15% greater relative biceps femoris activation compared to the normal group. The combination of decreased gluteus maximus activation (60%, p < 0.05) and increased biceps femoris (15%, p > 0.05) activation resulted in a significantly smaller relative gluteus maximus : biceps femoris co‐activation ratio between groups. Those with normal hip flexor length displayed a co‐activation ratio of 2.30, indicating their gluteus maximus activation amplitude was more than 2 times greater than the biceps femoris. In contrast, the restricted group demonstrated a gluteus maximus : biceps femoris co‐activation ratio of 0.88, indicating relatively greater activity of the biceps femoris. These findings suggest that individuals with restricted hip flexor length achieve comparable hip extension moments via a decreased activation of the primary hip extensor muscle (gluteus maximus) and relatively greater activation of the secondary or synergistic hip extensor muscles (biceps femoris) compared to individuals with normal hip flexor length.
Hip extension strength did not differ between the restricted and normal groups. Gluteal muscle strength has been observed to influence activation of the gluteal musculature, as weaker individuals display greater muscle activation compared to stronger individuals during the same standardized task.44 Since muscle strength was similar, it is possible the differences in muscle activation were primarily due to differences in hip flexor muscle length between groups. Hip flexor muscle tightness may facilitate reciprocal inhibition and / or an abnormal resting muscle length of the gluteus maximus musculature underlying the observed lower gluteus maximus : biceps femoris co‐activation ratio in the restricted group.
The current findings indicate that limited hip flexor muscle length does not directly alter internal hip and knee extension moments. However, the observed muscle activation strategy in individuals with limited hip flexor muscle length suggests that these individuals exhibit relatively greater reliance on hamstrings musculature versus gluteus maximus to eccentrically control hip flexion during a controlled functional movement. The requirement for greater hamstrings muscle co‐activation may impart greater stress on the hamstrings, thus clinicians should be aware of a potential for increased risk of a hamstring muscle strain injury in those with hip flexor muscle tightness, characteristic of biomechanical overload of muscle tissue.30 In addition, greater hamstring muscle co‐activation may make those with hip flexor muscle tightness more susceptible to hamstring muscle fatigue during sport.
The hamstrings are the primary muscle group responsible for controlling anterior tibial translation and shear forces, thus protecting the anterior cruciate ligament.45 Fatigue of the hamstring muscles may allow for increased ACL loading and injury in those with hip flexor muscle tightness. Collectively, these results implicate that individuals with hip flexor muscle tightness may be at risk for injury to the hamstrings and / or ACL. As such, increasing activation of the gluteus maximus may reduce synergistic dominance of the hamstrings, thereby reducing the risks of future hamstrings or ACL injury. Furthermore, increasing hip extension ROM through manual therapies and stretching paradigms aimed at increasing hip flexor muscle length may increase activation of the gluteus maximus, and reduce the requirement for greater hamstring co‐activation.7,24 Additionally, greater activation of the gluteal musculature during functional tasks may have a protective effect against hip internal rotation and hip adduction, readily identifiable components of dynamic medial knee collapse observed during noncontact ACL injury events.46 Thus, clinicians should consider prescribing gluteal muscle strengthening exercises for individuals with restricted hip flexor muscle length in efforts to achieve greater activation of the gluteal musculature.49
This study was not without limitations. The sample included healthy, physically active female soccer athletes between the ages of 18 to 35 years, thus it is not clear if these findings are generalizable to other populations. In addition, data analysis was limited to the descent phase of a DLS. The lower extremity movements during the descent phase of a DLS are similar to deceleration motions during other more demanding and functional tasks associated with injury events. However, it is unclear if the findings are transferrable to other tasks. Yet, the DLS exemplifies a task that is a compromise between representative motion during athletic tasks and an easily controlled motion to identify the effects of a specific ROM limitation on lower extremity hip and knee neuromuscular control.
It should also be noted that muscle strength was assessed via a maximal voluntary isometric contraction, yet the hip extensor musculature often functions in both a concentric and eccentric manner during more demanding functional tasks. Furthermore, the study methodology evaluated hip extension strength of the gluteal muscle mass, and did not isolate hamstring muscle strength. Thus the comparison of clinical hip extension strength between groups is primarily focused on gluteal strength. Collectively, it is unclear if the strength measures, though clinically relevant, adequately reflect the functional demands of the hip extensors. Finally, this study's methodology did not assess the activation of the primary hip flexor muscles, iliacus, and psoas major. Thus, it is not possible to determine if there was truly greater activation of the hip flexor muscles, which may have produced reciprocal inhibition of the gluteus maximus muscle in the restricted group. Future research should consider these limitations.21,47,48
Conclusions
In conclusion, individuals with restricted hip flexor length displayed hip and knee extension moments similar to those with normal muscle length, but they achieved these moments with decreased gluteus maximus activation and greater relative hamstrings co‐activation (reduced gluteus maximus : hamstrings co‐activation ratio). This decrease in gluteus maximus activation may be due to reciprocal inhibition of the gluteus maximus, which resulted in compensatory greater levels of relative hamstrings co‐activation to achieve the same internal hip extension moment. These findings suggest that hip flexor muscle tightness may be an important factor to consider in hamstring and ACL injury prevention programs. The findings of this study provide rationale for clinicians to consider implementing a treatment paradigm aimed at increasing hip extension range of motion and gluteal muscle strength.49 Achieving or maintaining normal hip flexor muscle length may decrease the potential for an inhibitory effect of the shortened hip flexors on gluteal neuromuscular control.
REFERENCES
- 1.de Loës M Dahlstedt LJ Thomée R. A 7‐year study on risks and costs of knee injuries in male and female youth participants in 12 sports. Scand J Med Sci Sports. 2000;2:90‐97. [DOI] [PubMed] [Google Scholar]
- 2.Cumps E Verhagen E Annemans L, et al. Injury rate and socioeconomic costs resulting from sports injuries in Flanders: data derived from sports insurance statistics 2003. Br J Sports Med. 2008;9:767‐772. [DOI] [PubMed] [Google Scholar]
- 3.Caine D Maffulli N Caine C. Epidemiology of injury in child and adolescent sports: injury rates, risk factors, and prevention. Clinics in Sports Medicine. 2008;1:19–50–vii. [DOI] [PubMed] [Google Scholar]
- 4.Toth AP Cordasco FA. Anterior cruciate ligament injuries in the female athlete. J Gend Specif Med. 2001;4:25‐34. [PubMed] [Google Scholar]
- 5.Marshall SW Padua DA McGrath M. Incidence of ACL injury. In: Hewtt TE Shultz SJ Griffin LY eds. Understanding and Preventing Noncontact ACL Injury. Champaign, IL: Human Kinetics; 2007:5‐29. [Google Scholar]
- 6.Cross KM Gurka KK Saliba S, et al. Comparison of hamstring strain injury rates between male and female intercollegiate soccer athletes. Am J Sports Med. 2013;4:742‐748. [DOI] [PubMed] [Google Scholar]
- 7.Mendiguchia J Alentorn‐Geli E Brughelli M. Hamstring strain injuries: are we heading in the right direction? Br J Sports Med. 2012;2:81‐85. [DOI] [PubMed] [Google Scholar]
- 8.Hägglund M Waldén M Magnusson H, et al. Injuries affect team performance negatively in professional football: an 11‐year follow‐up of the UEFA Champions League injury study. Br J Sports Med. 2013;12:738‐742. [DOI] [PubMed] [Google Scholar]
- 9.Esteve E Rathleff MS Bagur‐Calafat C, et al. Prevention of groin injuries in sports: a systematic review with meta‐analysis of randomised controlled trials. [published ahead of print March 27, 2015] Br J Sports Med. doi:10.1136/bjsports‐2014‐094162. [DOI] [PubMed] [Google Scholar]
- 10.Fousekis K Tsepis E Poulmedis P, et al. Intrinsic risk factors of non‐contact quadriceps and hamstring strains in soccer: a prospective study of 100 professional players. Br J Sports Med. 2011;9:709‐714. [DOI] [PubMed] [Google Scholar]
- 11.Krosshaug T Nakamae A Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;3:359‐367. [DOI] [PubMed] [Google Scholar]
- 12.Padua DA Marshall SW Beutler AI, et al. Sex Comparison of Jump Landing Kinematics and Technique. Med Sci Sports Exerc. 2004;S348. [Google Scholar]
- 13.Zazulak BT Ponce PL Straub SJ, et al. Gender comparison of hip muscle activity during single‐leg landing. J Orthop Sports Phys Ther. 2005;5:292‐299. [DOI] [PubMed] [Google Scholar]
- 14.Boden BP Torg JS Knowles SB, et al. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;2:252‐259. [DOI] [PubMed] [Google Scholar]
- 15.Kolber MJ Fiebert IM. Addressing Flexibility of the Rectus Femoris in the Athlete With Low Back Pain. Strength and Conditioning Journal. 2005;5:66‐73. [Google Scholar]
- 16.Krivickas LS Feinberg JH. Lower extremity injuries in college athletes: Relation between ligamentous laxity and lower extremity muscle tightness. Arch Phys Med Rehabil. 1996;11:1139‐1143. [DOI] [PubMed] [Google Scholar]
- 17.Teyhen D Bergeron MF Deuster P, et al. Consortium for health and military performance and American College of Sports Medicine Summit: utility of functional movement assessment in identifying musculoskeletal injury risk. Curr Sports Med Rep. 2014;1:52‐63. [DOI] [PubMed] [Google Scholar]
- 18.Ferber R Kendall KD McElroy L. Normative and critical criteria for iliotibial band and iliopsoas muscle flexibility. J Athl Train. 2010;4:344‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delp SL Hess WE Hungerford DS, et al. Variation of rotation moment arms with hip flexion. J Biomech. 1999;5:493‐501. [DOI] [PubMed] [Google Scholar]
- 20.Zeller BL McCrory JL Kibler WB, et al. Differences in kinematics and electromyographic activity between men and women during the single‐legged squat. Am J Sports Med. 2003;3:449‐456. [DOI] [PubMed] [Google Scholar]
- 21.Winters MV Blake CG Trost JS, et al. Passive versus active stretching of hip flexor muscles in subjects with limited hip extension: a randomized clinical trial. Phys Ther. 2004;9:800‐807. [PubMed] [Google Scholar]
- 22.Chumanov ES Wille CM Michalski MP, et al. Changes in muscle activation patterns when running step rate is increased. Gait Posture. 2012;2:231‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabbe BJ Bennell KL Finch CF. Why are older Australian football players at greater risk of hamstring injury? J Sci Med Sport. 2006;4:327‐333. [DOI] [PubMed] [Google Scholar]
- 24.Opar DA Williams MD Shield AJ. Hamstring strain injuries: factors that lead to injury and re‐injury. Sports Med. 2012;3:209‐226. [DOI] [PubMed] [Google Scholar]
- 25.Moor MA Hutton RS. Electromyographic investigation of muscle stretching techniques. Med Sci Sports Exerc. 1980;5:322‐329. [PubMed] [Google Scholar]
- 26.Alter MJ. Science of Flexibility. Champaign, IL;Human Kinetics; 2004. [Google Scholar]
- 27.Liebenson C. Rehabilitation of the Spine: a Pracitioner's Manual. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 28.Wagner T Behnia N, Ancheta W‐KL, et al. Strengthening and neuromuscular reeducation of the gluteus maximus in a triathlete with exercise‐associated cramping of the hamstrings. J Orthop Sports Phys Ther. 2010;2:112‐119. [DOI] [PubMed] [Google Scholar]
- 29.Sahrmann S. Diagnosis and Treatment of Movement Impairment Syndromes. Oxford, UK: Elsevier Health Sciences; 2013. [Google Scholar]
- 30.Franklyn‐Miller A Roberts A Hulse D, et al. Biomechanical overload syndrome: defining a new diagnosis. Br J Sports Med. 2014;6:415‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renstrom P Peterson L. Groin injuries in athletes. Br J Sports Med. 1980;1:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renström P Johnson RJ. Overuse Injuries in Sports A Review. Sports Med. 1985;5:316‐333. [DOI] [PubMed] [Google Scholar]
- 33.Ford KR Myer GD Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;10:1745‐1750. [DOI] [PubMed] [Google Scholar]
- 34.McLean SG Huang X van den Bogert AJ. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: implications for ACL injury. Clin Biomech. 2005;8:863‐870. [DOI] [PubMed] [Google Scholar]
- 35.Pantano KJ White SC Gilchrist LA, et al. Differences in peak knee valgus angles between individuals with high and low Q‐angles during a single limb squat. Clin Biomech. 2005;9:966‐972. [DOI] [PubMed] [Google Scholar]
- 36.Clapis PA Davis SM Davis RO. Reliability of inclinometer and goniometric measurements of hip extension flexibility using the modified Thomas test. Physiother Theory Pract. 2008;2:135‐141. [DOI] [PubMed] [Google Scholar]
- 37.Gabbe BJ Bennell KL Wajswelner H, et al. Reliability of common lower extremity musculoskeletal screening tests. Physical Therapy in Sport. 2004;2:90‐97. [Google Scholar]
- 38.Peeler J Anderson JE. Reliability of the Thomas test for assessing range of motion about the hip. Physical Therapy in Sport. 2007;1:14‐21. [Google Scholar]
- 39.Rainoldi A Melchiorri G Caruso I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J Neurosci Methods. 2004;1:37‐43. [DOI] [PubMed] [Google Scholar]
- 40.Bell AL Pedersen DR Brand RA. A comparison of the accuracy of several hip center location prediction methods. J Biomech. 1990;6:617‐621. [DOI] [PubMed] [Google Scholar]
- 41.Grood ES Suntay WJ. A joint coordinate system for the clinical description of three‐dimensional motions: application to the knee. J Biomech Eng. 1983;2:136‐144. [DOI] [PubMed] [Google Scholar]
- 42.Winter DA. Biomechanics and Motor Control of Human Movement. 4 ed. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 43.Shimokochi Y Ambegaonkar JP Meyer EG, et al. Changing sagittal plane body position during single‐leg landings influences the risk of non‐contact anterior cruciate ligament injury. Knee Surg Sport Tr A. 2013;4:888‐897. [DOI] [PubMed] [Google Scholar]
- 44.Homan KJ Norcross MF Goerger BM, et al. The influence of hip strength on gluteal activity and lower extremity kinematics. J Electromyogr Kinesiol. 2013;2:411‐415. [DOI] [PubMed] [Google Scholar]
- 45.Withrow TJ Huston LJ Wojtys EM, et al. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008;4:815‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krosshaug T Slauterbeck JR Engebretsen L, et al. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three‐dimensional motion reconstruction from video sequences. Scand J Med Sci Sports. 2007;5:508‐519. [DOI] [PubMed] [Google Scholar]
- 47.Di Giulio I Maganaris CN Baltzopoulos V, et al. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol (Lond). 2009;587:2399‐2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrissey D Graham J Screen H, et al. Coronal plane hip muscle activation in football code athletes with chronic adductor groin strain injury during standing hip flexion. Man Ther. 2012;2:145‐149. [DOI] [PubMed] [Google Scholar]
- 49.Reiman MP Bolgla LA Loudon JK. A literature review of studies evaluating gluteus maximus and gluteus medius activation during rehabilitation exercises. Physiother Theory Pract. 2012;28:257‐268. [DOI] [PubMed] [Google Scholar]