Abstract
The degree to which non-human primate behavior is lateralized, at either individual or population levels, remains controversial. We investigated the relationship between hand preference and posture during tool use in chimpanzees (Pan troglodytes) during bipedal tool use. We experimentally induced tool use in a supported bipedal posture, an unsupported bipedal posture, and a seated posture. Neither bipedal tool use nor these supported conditions have been previously evaluated in apes. The hypotheses tested were 1) bipedal posture will increase the strength of hand preference, and 2) a bipedal stance, without the use of one hand for support, will elicit a right hand preference. Results supported the first, but not the second hypothesis: bipedalism induced the subjects to become more lateralized, but not in any particular direction. Instead, it appears that subtle pre-existing lateral biases, to either the right or left, were emphasized with increasing postural demands. This result has interesting implications for theories of the evolution of tool use and bipedalism, as the combination of bipedalism and tool use may have helped drive extreme lateralization in modern humans, but cannot alone account for the preponderance of right-handedness.
Keywords: Bipedalism, Posture, Handedness, Laterality, Asymmetry, Tool use, Chimpanzee, Hand preference
Introduction
One characteristic that distinguishes humans from other primates is that a majority of humans, close to 90%, are right-handed (Gilbert and Wysocki, 1992; Perelle and Ehrman, 1994). A bias of this magnitude has not been found in any other primate species. Despite considerable disagreement as to how handedness should be defined or measured, the handedness of multiple primate species has been evaluated in a variety of tasks. Handedness is one component of the concept of laterality (having a behaviorally dominant side or limb), often presumed to be indicative of asymmetry of the brain (Heestand, 1986; Hopkins and Morris, 1993; Hopkins, 2007). Laterality can be evaluated by determining which side of the body has more control relative to the other, or by determining which side of the brain is more responsible for specific actions or behaviors. Individual laterality and side preferences have been shown in many species, including rats, chickens, elephants, whales, and even snakes for slithering direction (Walker, 1980; Rogers, 1989; Rogers and Workman, 1993; Clapham et al., 1995; Bisazza et al., 1998; Martin and Niemitz, 2003).
Primates and other vertebrate species show laterality of function, but no other primate species shows such a marked or extensive cerebral asymmetry at a population level as humans (Vallortigara and Rogers, 2005). Therefore, laterality is often thought to have played an important role in the evolution of human cognition. Speech is typically lateralized to the left hemisphere of the human brain, but can occasionally be expressed in the right hemisphere (Knecht et al., 2000a,b). Apes do not exhibit spoken language, but if they do display laterality, it probably reflects a trait present in the last common ancestor of humans and other great apes, and this trait may have acted as a pre-adaptation in the evolution of language (Hopkins and Cantero, 2003; Vallortigara and Rogers, 2005; Steele and Uomini, 2009). Other lateralized behaviors hypothesized to have influenced the evolution of cognition include tool use (Gibson and Ingold, 1993; Preston, 1998), manual gestures (Hopkins and Leavens, 1998; Rizzolatti and Arbib, 1998; Corballis, 2003; Pollick and de Waal, 2007), and throwing (Hopkins et al., 1993, 2005a,b). Furthermore, posture has been shown in some previous studies to influence handedness (Roney and King, 1993, Hopkins and Morris, 1993), with upright or bipedal postures increasing right-handedness, suggesting a need to evaluate the effects of tool use and bipedal posture concurrently.
The aim of the present study is to examine the relationship between hand preference and posture during a tool use task in captive chimpanzees. We manipulated the task demands so that the tool-use could be accomplished 1) while seated, 2) while bipedal but with one hand against a wall, and 3) while fully bipedal. The main goals were to test the prediction that assumption of bipedal posture would increase the strength of right-hand hand preference during tool use. Based on the existing literature, we tested two specific hypotheses: H1) bipedal posture increases the strength of hand preference, without respect to side, and H2) more specifically, a bipedal stance, without the use of one hand for support, elicits a right hand preference.
These two hypotheses need to be distinguished because previous work in nonhuman animals show that a group of animals may differ in laterality overall (that is, some animals in a group may be ambidextrous, while others are strongly lateralized, but with equal numbers of left- and right-lateralized individuals). Such lateralized species might still lack any group- or population-level directional bias to use the right hand. Humans, of course, are both lateralized (ambidextrous individuals are rare) and directionally lateralized to the right side (left-handed individuals are equally rare), but these two characteristics need not go together. We distinguish between these two logical possibilities by calculating, for each subject, both a handedness index (HI; ranging from 1.0 to −1.0, and whose sign reveals the directional bias to the right or left respectively) and an absolute handedness index (ranging from 0.0 for ambidextrous, to 1.0 for strongly lateralized animals which use either the left or right hand exclusively).
Methods
Subjects
For this experiment, 46 chimpanzees (28 males and 18 females) ranging in age from 12 to 47 years (mean age of 28.15 years) of various subspecies (mostly Pan troglodytes verus) were used. The chimpanzees are housed at the Michale E. Keeling Center for Comparative Medicine and Research at The University of Texas M.D. Anderson Cancer Center in Bastrop, Texas (MDACC), and research was conducted with relevant IACUC approvals. The facility has eight open top corrals, each providing both indoor and outdoor housing to 7–14 animals per group. All chimpanzees remained in their home corrals for testing. Subjects were chosen from all corrals to be included in all experimental conditions. Subjects were selected based on their handedness in previous studies and included 15 right-handed, 16 left-handed, and 15 ambidextrous individuals (Hopkins et al., 2003). These animals all have considerable experience extracting food from tubes, due to both frequent enrichment (pipe feeders are provided on a weekly basis which require tools to be inserted into fixed pipes to extract various food substances) and previous exposure to a similar task (Hopkins et al., unpublished data).
Materials
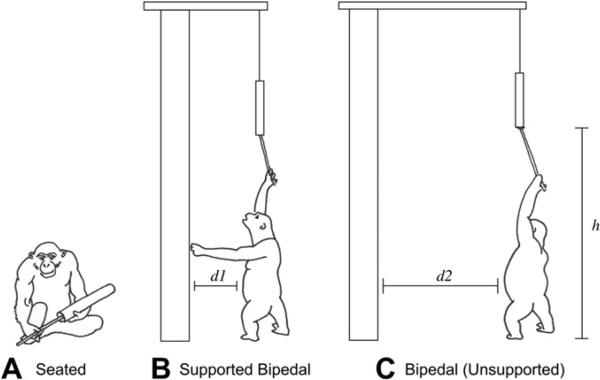
A poly-vinyl-chloride (PVC) tube (135 cm in length, 4 cm in diameter) with peanut butter in the center was suspended in an outdoor enclosure using 80 lb test fishing line connected to an eyelet in the cap of the tube. Fishing line was used so that the line broke each time a chimpanzee grabbed hold of the food tube and pulled downwards, ensuring that the animals could not climb up the line and escape their enclosure. In the event that a chimpanzee jumped and grabbed the tube, the researcher returned to ground level and recovered the tube, cap, and broken line. In order to better maintain a consistent distance, the fishing line was strung through a 1.35 m PVC tube and secured (Fig. 1). The food tube was lowered into the enclosure until it was approximately 2.8 m off the ground, which is the total of the average height of an adult chimpanzee (150 cm), the average length of a chimpanzee arm (83 cm), and the length of the tool (45 cm). The distance of the food tube from the interior walls of the corral differed based on experimental condition.
Fig. 1.
Peanut butter tube suspension apparatus viewed from the side. The tube about to be put into the corral is in front of the suspension system and the fishing line is run through the larger PVC and wrapped around the top extension to secure it.
Procedures
All subjects participated in all three of the experimental conditions, first in the seated condition (data collected in 2002), then the supported bipedal conditional, the bipedal condition, and finally in a retest of the seated condition. The initial seated data were used to allocate individuals to three groups of equal size (of left- handed, right-handed, and ambidextrous individuals). For all conditions, trials were run daily, with a minimum of 36 hours between trials for any particular group. All trials took place in the outdoor section of the subject's home corral and subjects from each corral were tested. Research in a particular corral lasted at least 2 hours in order for all focal animals to have the opportunity to gain access.
Each trial, regardless of condition, began with the researcher placing cut bamboo sticks (45 cm long) within reach of every member of the test group. These tools were then gathered by the subjects, without any restriction on the hand used to take the stick. PVC tubes with peanut butter smeared in the center (near the midpoint of the tube's axis) were provided to the chimpanzees along with tools in the form of the cut bamboo sticks. Peanut butter was placed only in the center of the tube to encourage tool use and prevent subjects from using their hands to extract the peanut butter.
We recorded data for all sessions on a dictaphone via spoken commentary. We use the term ‘event’ to designate one instance of feeding (e.g., inserting the tool into the tube, pulling out the tool, inserting the tool into the mouth, and repeating) and trials continued until the focal subjects in each group had displayed at least 50 events, over a minimum of three testing trials. “Bouts” were groups of events, which either occurred on different days, or in which the subject put down the tool, left the test apparatus, and later returned during a single test session. “Bout-wise” data were scored using only the first event of each bout as independent data points, while “event-wise” analyses incorporated all events as data points. In order to meet the designated minimum of 50 events, between 3 and 11 data collection trials were completed by the chimpanzees, during which the chimpanzees completed 50 to 72 events. Between 3 and 11 (mean of 4.95) independent ‘bouts’ were scored as single data points for the bout-wise HI.
Handedness Indices (HI) for all animals were calculated to quantify the degree of lateral bias. This was done by subtracting the number of left hand uses (L) from the number of right hand uses (R), and dividing by the total number of hand use instances (R +L):
Handednes Index values range from 1.0 (extreme right-handed) to −1.0 (extreme left-handed). Absolute Handedness (AbsHI) was also calculated (absolute HI = |HI|); the absolute value of the HI score represents the strength of hand preference irrespective of direction and ranges from 0 (ambidextrous) to 1.0 (extreme lateralization in either direction). Statistical analysis was conducted using JMP software version 8.0.1 and SPSS software version 16.
We experimentally manipulated our subjects’ posture in the following three conditions:
Seated condition (SC)
This condition was run twice, first to assign animals to groups, and again after all other trials, in order to reassess baseline hand preference. In this situation the chimpanzee was allowed to hold the food tube and all chimpanzees sat down before extracting the peanut butter (Fig. 2A).
Fig. 2.
Sketch of a chimpanzee performing the task for each condition. A) Baseline condition: seated tool use. B) Supported bipedal condition: bipedal tool use while using one arm for support. C) Bipedal condition: completely bipedal tool use without any support. Measurements indicated are d1: 75 cm maximum, d2: 100 cm minimum, h: 2.8 m.
Supported bipedal condition (SB)
From the roof of the corral, the food tube was positioned within reaching distance of the coral wall (approximately 75 cm away), allowing the subject to use one hand to perform the tool use task and the other to provide postural support by bracing themselves against the wall (Fig. 2B). The researcher remained on the roof for the duration of the trial in order to lower and raise the apparatus as needed in order to prevent the tubes from being pulled into the enclosure.
Bipedal condition (B)
The methods used for this condition were identical to that of the supported bipedal condition test, with only one exception. For this experimental condition, the tube was suspended approximately 1 m away from the wall so that the subjects could not use their hand to support themselves against the wall, forcing them to adopt an unsupported bipedal posture (Fig. 2C).
Results
We found no significant deviation from normality (Kolmogorov-Smirnoff test, p > 0.05 for all conditions), for either Handedness Index (HI) or the absolute HI (AbsHI). Thus we used parametric ANOVAs to examine these data.
Strength of hand preference
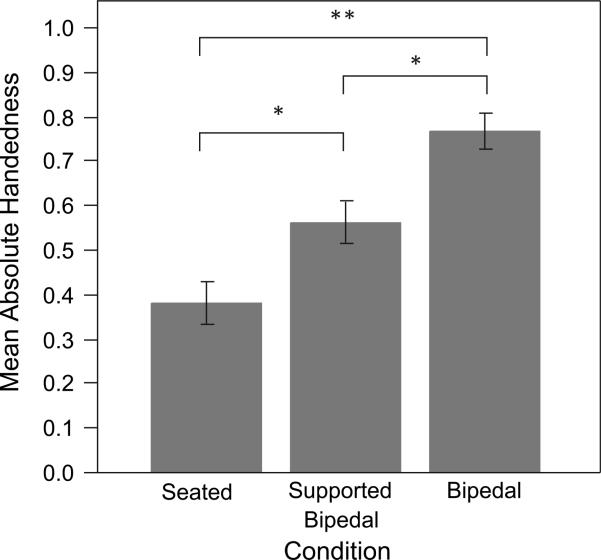
To evaluate the effect of posture on the strength of hand preference, we examined the absolute values of each subject's HI, as calculated from each event (Fig. 3). A repeated measures ANOVA with postural condition as the independent variable and the absolute value of the HI as the dependent variable yielded a significant main effect for postural condition (F [2, 44] = 37.012, p < 0.001). A Tukey post hoc analysis indicated that the mean absolute HI significantly increased from the seated condition to the supported bipedal condition (p = 0.019), from the supported to the bipedal condition (p = 0.004), and from the seated to the bipedal condition (p < 0.001). A non-parametric Wilcoxon signed ranks test was also performed with identical results (seated to supported condition z = −2.71, p = 0.007; supported to bipedal condition z = −3.64, p < 0.001; seated to bipedal condition z = −4.76, p < 0.001).
Fig. 3.
Mean absolute value of HI with error bars indicating mean standard error. The changes in mean absolute value of HI between the baseline and the supported condition was significant (*p < 0.02), as was that between the supported condition and the unsupported condition (*p < 0.01), and between the baseline and the unsupported bipedal posture (**p < 0.001).
Categorized effects
The subjects used here were selected based on their handedness category from an unpublished previous study conducted in 2002. To test for stability across time, the handedness results from the 2002 study were correlated with the current results from our seated condition. The HI from the 2002 study is very strongly correlated with the HI from the current seated condition (r2 = 0.70; p < 0.0001). Every animal but one showed nearly identical HI across this five-year period; a single female switched from strongly right to strongly left-biased. When this female is removed from the analysis, the correlation across studies becomes nearly perfect (r2 = 0.98; p < 0.0001). Thus, individuals are very stable in their performance of this task.
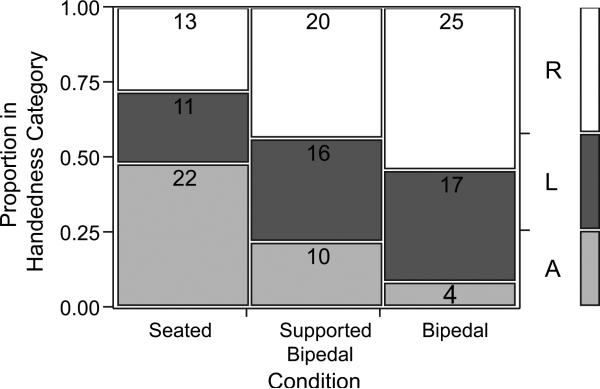
It is common in the literature to classify subjects as left-handed, right-handed, or ambidextrous, based on the number of right and left hand responses. For comparison with other studies, subjects with a z-score equal to or greater than 1.96 were considered right-handed, and subjects with a z-score equal to or less than −1.96 were considered left-handed (Fig. 4). Subjects with z-score values between these (−1.96 < z < 1.96) were considered to be ambidextrous, reflecting the common practice used in the nonhuman primate literature (see Hopkins,1999). The distributions of these handedness values differed significantly between conditions, when including all three postures (right-handed, left-handed, and ambidextrous) (χ2[4, n = 138] = 19.599, p < 0.001), but when only the right-handed and left-handed categories are examined, the conditions no longer differ significantly (χ2 [2, n = 102] = 0.218, p > 0.05). This is because as the task became more bipedal, the number of ambidextrous subjects dissolved into either right- or left-handed categories (Fig. 4).
Fig. 4.
Number of right-handed (R), left-handed (L), and ambidextrous (A) subjects by condition. Note the steady decrease in the number of ambidextrous subjects as the task demanded increasing bipedality, and roughly equivalent numbers of right- and left-handed animals with a slight bias towards right-handedness.
A repeated measures analysis of variance (ANOVA) was performed with experimental condition as the independent variable and HI as the dependent variable. No main effect was found (F [2, 44] = 0.762, p = 0.473). This was confirmed with a Wilcoxon signed rank test. The increase in right-handedness in the classified data and decrease in number of subjects showing no hand preference is not significant, as a result of high variance.
Bouts versus events
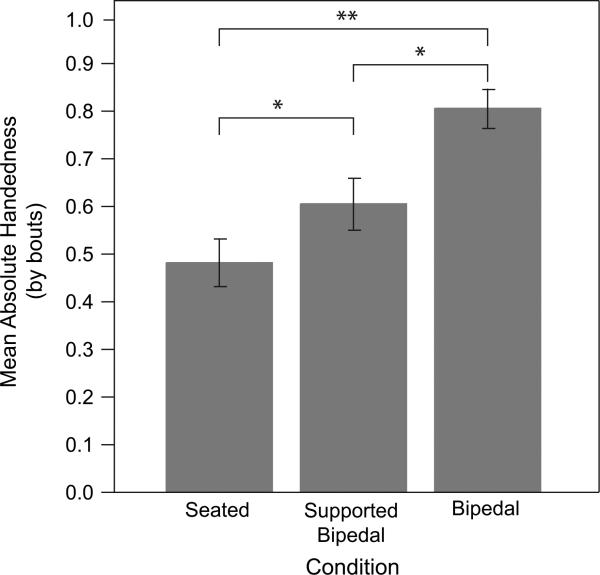
To address potential confounds to the independence of the handedness measures (McGrew and Marchant, 1997), we reanalyzed data in terms of bouts (see Methods) and compared it to the previous results. First, a significant correlation was found between HI measured with events and HI using bouts (r = 0.714, p < 0.001). The normality of the bout-oriented data was tested using the Kolmogorov-Smirnov goodness-of-fit test for conditions 1 and 2 (p > 0.05), but the bipedal condition showed a significant (p = 0.03) departure from normality for both HI and AbsHI. Thus, a Wilcoxon matched-pairs signed ranks test was performed to compare HI in the seated condition to the supported condition, the supported condition to the bipedal condition and the seated condition to the bipedal condition. As before, absolute values of bout-wise HI differed significantly among conditions (seated to supported condition z = 2.47, p = 0.013; supported to bipedal condition z = −3.80, p < 0.001; seated to bipedal condition z = −4.47, p < 0.001) (Fig. 5). Subjects increased their strength of hand preference as the task demanded more bipedality. However, as before, HI alone showed no significant change across conditions, showing no trend towards right- or left-handedness with increasing bipedality.
Fig. 5.
Mean absolute value of HI when re-examined per “bout” with error bars indicating mean standard error. All changes were significant: between the baseline and the supported condition (*p < 0.02), between supported and unsupported bipedal (**p < 0.001), and between baseline and unsupported bipedal (**p < 0.001).
Discussion
This study is the first to include tool use in an examination of posture and hand preference in a large population of chimpanzees. Our data show that during tool use in a bipedal posture, chimpanzee hand preferences become more lateralized. More specifically, as posture became less stable (from a seated to a supported bipedal stance to an unsupported bipedal posture), a significant increase in absolute handedness was observed. Subjects were most strongly lateralized when standing upright in an unsupported bipedal stance, compared to a supported bipedal stance or a seated posture, and were also more strongly lateralized when in the supported bipedal stance compared to a seated posture. These data strongly support our first hypothesis, that bipedal posture should increase lateral asymmetry, and the strength of hand preference toward either the right or left hand. However, a slight apparent bias towards being more right-handed as posture became bipedal was not significant, providing no support for our second hypothesis. This slight bias towards right-handedness may warrant further exploration, but our data do not support the suggestion that bipedal tool use drives most individuals towards right-handedness.
Our finding has a number of implications regarding the evolution of laterality in humans, along with both theoretical and methodological implications for the study of laterality in other animals. We start by discussing the contribution of these data to current understanding of primate laterality in general, before turning to issues concerning human evolution.
Posture, tool use and lateralization in nonhuman primates
Various studies suggest that handedness in great apes may be linked to posture and/or tool use (Olson et al., 1990; Hopkins, 1993; Hopkins et al., 2007; Cantalupo et al., 2008). Several previous studies report a right-hand bias when in bipedal versus quadru-pedal posture in all four great ape species, but none of them directly examined bipedal tool use. The results of the current study differ because chimpanzees show a significant preference toward right-handedness for bipedal tasks (Hopkins and Morris, 1993). This disparity could be a consequence of differences in the methods employed. For example, the previous studies did not use a seated posture as a baseline measure to preselect their subjects, nor did they examine tool use, or experimentally manipulate the degree of support.
From a methodological viewpoint, our results provide no support for the suggestion of McGrew and Marchant (1997) that bout-wise analyses provide a superior measure of hand preference, or that the findings of Hopkins et al. (2001) using a different population of chimpanzees, result from a statistical artifact of event-wise measures. With our individuals, and relatively large sample sizes, bout-wise and event-wise measures are very strongly correlated, so neither measure is clearly preferable.
Nonhuman primate hand preferences have rarely been examined in light of species differences in morphology (Preuschoft and Chivers, 1993; Bradshaw and Rodgers, 1993), although grip morphology in relation to hand preference has been explored in chimpanzees (Hopkins et al., 2002). In contrast, morphology and skeletal asymmetry has been closely scrutinized in fossil hominins in order to shed light on the evolution of handedness in early humans (Lazenby, 2002; Cashmore et al., 2008). Some species differences may result from environmental or ecological factors (e.g., arboreality) that indirectly influence hand preference through posture. Primates such as bushbabies (Larson et al., 1989), ruffed lemurs (Forsythe et al., 1988), and gibbons (Olson et al., 1990) have exhibited non-population level left-hand preferences for bipedal food reaching (cf. MacNeilage et al., 1987). These species are more highly arboreal than chimpanzees, and perhaps require more visual spatial guidance, a function hypothesized to rely preferentially on the right hemisphere of the brain (Maravita and Iriki, 2004). However, bonobos also rely on arboreal locomotion more than chimpanzees and appear to spend more time in trees, but show no consistent handedness (Doran, 1993; D'Août et al., 2004; Harrison and Nystrom, 2008). In gorillas, (Byrne and Byrne, 1991) individual-level asymmetry in complex foraging tasks was often quite pronounced, but no population-level bias existed. These gorilla results are thus quite similar to our own experimental findings.
The chimpanzees tested in our study became more lateralized (asymmetrical) as a result of experimentally-induced changes in posture. As posture shifted from a familiar and relaxed seated posture to a less stable bipedal posture, the strength of hand preference increased as well. These results are congruent with those of Roney and King (1993) where cotton top tamarins and squirrel monkeys displayed higher levels of laterality while in a vertical clinging posture as compared to a quadrupedal posture.
Postural origins theory
After a long period of belief that no population-level laterality exists in primates, a seminal paper reexamined the data (MacNeilage et al., 1987), concluding that slight but significant preferences existed in many primates at the species level, which led to the “Postural Origins Theory” of human handedness (POT). The POT suggests that a basic lateralization to the left hand for grasping exists in many primates, leaving the right hand to support the body in quadrupedal primates. With the assumption of upright bipedalism, the human right hand was freed from this traditional role in support, and used to perform fine manipulations on the object grasped by the left hand. Thus, the human right hand became specialized for tool use (MacNeilage, 1991).
Our finding that posture has an effect on asymmetry lends partial support to the POT, which suggests that as primates became less arboreal, their postures shifted to reflect the most efficient feeding methods (MacNeilage et al., 1987). However, the research presented here does not support the proposal of the postural origins theory that there should be an overall shift to a right-hand tool preference, for example, due to the fine hand-eye precision required to insert a tool into a tube while standing bipedally.
Laterality, arousal, and complexity
Our study supports the idea that hand preference may increase with arousal in the central nervous system (Larson et al., 1989; Westergaard et al., 1998), and for Fagot and Vauclair's (1991) theory that high-level or difficult tasks reflect specializations in the brain better than simple low-level tasks. The bipedal tool use task, especially without any support, was an uncomfortable and somewhat taxing task for the chimpanzee subjects in this study. The bipedal posture appeared to be difficult for the chimpanzee subjects as evidenced by shaking legs during the task and resistance on the part of some animals to perform the bipedal task at all. Thus, the difficulty of maintaining a stable bipedal posture while performing a fine task may drive our chimpanzees to be more consistent in the hand they use to manipulate the tool in this task.
Our task allowed us to separate the effects of difficulty from those arising from the specific complexity of tool use. Steele and Uomini (2009) correctly observe that discussions of “manual dominance” often forget that in most tool making and tool use tasks the left hand plays an important role in positioning or stabilizing the target. Similarly, Rogers (2009) notes that discussions of “complexity” of tasks used in handedness research ignore the fact that manual tasks may reflect the “processing styles” of the two cerebral hemispheres (e.g., for spatial relations versus fine detail), rather than complexity per se. Thus, human tool use is often better characterized by a division of labor among the hands, rather than simple “dominance” of one over the other. These criticisms, while often well-founded, do not apply in our study because in bipedal conditions, the non-tool using hand is only involved in postural stabilization and not in any aspect of tool use. Thus, our task cleanly separates the roles of difficulty from the complementary or synergistic use of the two hands, and shows that bipedalism drives increased laterality independent of any such synergy.
Implications for the evolution of human handedness
Discussions of the evolution of human handedness must cope with several seemingly contradictory observations. The first and most obvious is that humans show a degree of right-lateralization, roughly 90%, that is unparalleled in other primates (Gilbert and Wysocki, 1992; Perelle and Ehrman, 1994), especially in the context of tool use (Marchant et al., 1995; Stout, 2002). Although debate about right-laterality in chimpanzees will continue, all parties agree that any population-level bias that exists in this species is not nearly as strong as in humans— roughly 65% whether for throwing, tool use, or gestural communication (Hopkins, 1996; Hopkins and Leavens, 1998; Hopkins et al., 2005a). Furthermore, ambidexterity is common in chimpanzees but rare in humans (Hopkins, 2006). Field researchers have failed to find even this level of right bias in wild populations (Sugiyama et al., 1993; McGrew and Marchant, 1997), although Lonsdorf and Hopkins (2005) report a population-level bias for termite fishing in wild chimpanzees. Any complete theory of handedness evolution will have to account for a major quantitative shift in right-lateralization in the human lineage.
Despite the human species-typical right bias, a stable and significant number of humans are left-lateralized, and the evidence available suggests that this polymorphism has existed for many tens of thousands of years (Coren and Porac, 1977; Steele and Uomini, 2005; Llaurens et al., 2009). This illustrates that left-handedness is perfectly compatible with successful existence as a human, and suggests that it has some advantage(s), apparently balancing the selection for right-handedness which might otherwise have driven humans to 100% right-handedness. While many hypotheses for this “balancing advantage” have been discussed (cf. Llaurens et al., 2009), the most likely seems to be an advantage enjoyed by left-handers in fighting and other physical competition (Porac and Coren, 1981; Annett, 1985; Ghirlanda et al., 2009).
Finally, cerebral asymmetry for language is even more pronounced than handedness. This is because, in addition to the 93% of right-handed humans left-dominant for language (Knecht et al., 2000a,b), most left-handers are also left-dominant. Only a small proportion (around 10% of 50 left-handed subjects) show true mirror-reversal and complete right-hemisphere dominance (Pujol et al., 1999). Cerebral asymmetry for language does not co-assort perfectly with handedness, and is both stronger than, and somewhat independent of, the motor asymmetry underlying handedness.
Cerebral asymmetry and handedness
While cerebral asymmetry was long argued to be a distinctive feature of humans (e.g., Geschwind, 1970; Corballis, 1983; Annett, 1985; Crow, 2004), recent data overwhelmingly support the idea that cerebral asymmetry is widespread among diverse vertebrates for certain functions, notably communication and social behavior (cf. Rogers and Andrew, 2002; Vallortigara, 2006; Rogers, 2009). This strong evidence contrasts sharply with the much weaker evidence for population-level handedness bias among nonhuman animals, and strongly suggests that handedness and cerebral lateralization for language, though often conflated, need to be clearly distinguished in discussions of the evolution of laterality.
Our data support the need for this distinction, and suggest a two-component (and perhaps two-stage) model of the evolution of human lateralization. First, our finding that the combination of tool use and bipedal posture drives chimpanzees to be more lateralized, but in a random direction, suggests that bipedal hominins from Australopithecus onward would have had more pronounced individual-level lateral asymmetries, at least during tool use, than those observed in modern chimpanzees. Many authors have proposed functional advantages to cerebral asymmetry in either direction, including the avoidance of unnecessary duplication of neural circuitry (Levy, 1977), and efficient parallel processing by the two hemispheres (Rogers, 2002). Our data are clearly compatible with the idea that a strengthening of asymmetry occurred and provided selective advantages quite early in hominin evolution. Because basic tool use characterizes both chimpanzees and humans, and was thus presumably present in the last common ancestor of these species, we hypothesize that a strengthening of individual asymmetry started as soon as early hominins assumed a habitual upright posture during tool use or foraging.
Language and handedness
We found no evidence from chimpanzees that posture and tool use would be enough to drive population-level right bias beyond a level seen in modern great apes, so some additional factor must be invoked to explain the strong right-bias found in all modern human populations. The most commonly-cited additional factor is language (Steele and Uomini, 2009), termed the “Homo loquens” hypothesis. For example, Corballis (2003) has suggested that humans’ extreme right bias for skilled action is better explained by lateralization for language than any specifically manual selective force. Population level right-handedness may represent an unselected byproduct, or “spandrel” of left-brainedness, selected for independent functional reasons. As noted above, this is supported by the fact that language is more lateralized than hand preferences because most left-handers are also left-lateralized (Pujol et al., 1999; Knecht et al., 2000b). An alternative possibility is that language and manual specialization co-localize because both rely on some kind of “syntactic” or rule-governed process, and thus lateralization based on tool-use preceded that for language (Bradshaw and Nettelton, 1982; see the “Homo faber” hypothesis of Steele and Uomini, 2009).
Our data provide significant support for “language first” scenarios, although not for any sharp discontinuity in the evolution of handedness (e.g., Crow, 2004). Our data clearly suggest that, although bipedality and tool-use may be enough to drive asymmetry in general (e.g., in early bipedal hominids like Australopithecus), the specific localization of hand dominance to the right side in all modern humans appears to require some other force. Language lateralization appears to be a promising candidate.
Why should language drive a population-level bias? One factor may be ancient population-level brain asymmetries present in many vertebrates. Overwhelming evidence has now accumulated indicating that neural and behavioral asymmetries, particularly at the sensory level, are a ubiquitous feature in vertebrates (Vallortigara and Rogers, 2005). Although many species show asymmetry at the individual level (Tsai and Maurer, 1930; Bradshaw and Rodgers, 1993; Cowell et al., 1997), population-level laterality is well-documented in a much smaller number of vertebrates, including toads (Bisazza et al., 1996) and cockatoos (Rogers and Workman, 1993; Rogers, 2007), and may be dependent upon sensory proclivities (Rogers, 2009). Although there is considerable variety in the species examined and means of perceptual testing employed, the left hemisphere is regularly more involved in practice- or experience-dependent behaviors, while the right hemisphere specializes in instinctive and spatial tasks (Güntürkün et al., 2000; Andrew and Rogers, 2002). In particular, socially-salient stimuli (e.g., recognition of conspecifics or sexual behavior) seem to be the preferential domain of the right hemisphere in many species. Thus biases for left-lateralization, seen in many vertebrates for social interaction, may have provided the seed for the evolution of pronounced human cerebral asymmetry for language. However, as already stressed, some other factor must be invoked to explain its extreme strength. One promising route for explaining the strengthening of a pre-existing left-bias is group coordination. Ghirlanda and colleagues have developed game-theoretic analyses showing that pressure for group coordination can lead to strong population-level biases in asymmetry (Ghirlanda and Vallortigara, 2004; Ghirlanda et al., 2009), and that such biases form an evolutionarily stable strategy against non-biased mutants. Because language use requires coordination among individuals in a social group, it is plausible that the evolution of language requires such a population-level bias. While the need for coordination does not itself explain a right- or left-bias, this additional selective pressure might have built upon the “seed” of pre-existing vertebrate sensory biases.
In summary, we find support in our data for what Steele & Uomini (2009) term the “Homo loquens” model of the evolution of human asymmetry, whereby individual asymmetry was driven by bipedal posture and tool use, but population-level asymmetry was driven, probably much later, by the evolution of language. In this second stage, the need for group coordination might have provided the selective force, as suggested by Ghirlanda et al. (2009), and preexisting vertebrate biases for social perception (Rogers, 2009) or weak primate biases for communication (Hopkins et al., 2005b) provided the “seed” that led to right-bias becoming the default state for all modern humans. A contravening force favoring left-handedness for independent reasons such as fighting or other physical competition has maintained a low but significant level of left-handedness ever since, in a stable polymorphism (Llaurens et al., 2009). This model integrates multiple existing models for the evolution of human handedness, and is consistent with theoretical models, data on human handedness and cerebral laterality, and the comparative data from both chimpanzees and a variety of other vertebrates. It also supports the long-standing suggestion that archaeological evidence for handedness might be used to try to date the origins of language.
In their thorough review of fossil and material-culture evidence for right handedness in hominin evolution, Steele and Uomini (2005) stress the tenuous nature of most of this evidence. Although early paleoanthropologists claimed evidence for handedness in the genus Australopithecus (Dart, 1949), most recent commentators have rejected these suggestions, and associate the earliest evidence for population-level handedness with the genus Homo (Steele and Uomini, 2005). Even in Homo, however, they found only a limited amount of evidence consistent with population-level right hand bias until anatomically modern humans, with the data for Neanderthals providing the strongest evidence outside of our own species. In an influential study at the Koobi Fora site, Toth (1985) found evidence for right-bias among flint knappers in Oldowan and Acheulean tool-making assemblages, but subsequent researchers have questioned the reliability of this archaeological signal (cf. Steele and Uomini, 2005). These data are also consistent with the two stage model sketched above, whereby strong individual-level asymmetries long preceded population-level hand preferences, later brought on by the evolution of language.
Data from experimental work on handedness in chimpanzees can directly inform discussions of the evolution of human handedness. Our data support the notion that the evolution of upright posture in early hominins had a direct and significant effect on levels of individual hand preference. Although the relatively simple tool-use task in our study involved only one hand, and thus has the virtue of clearly separating the roles of posture and task difficulty from tool use per se, future studies might profitably investigate the role of posture on more complex tool use tasks that involve both hands. Such tasks might be more relevant to human hand preference, which typically involves synergy between the “dominant” and non-dominant hands. Although our data do not resolve the long-running debate concerning population-level hand preferences in chimpanzees, they underscore the widely-recognized fact that any such preferences are quite weak in chimpanzees when compared to humans, and that they depend on the specific task chosen. Our data also clearly indicate that future studies need to pay careful attention to the posture assumed by primates when evaluating behavioral asymmetries, as posture can have a strong effect on the strength of asymmetries expressed. Finally, our results nicely illustrate the complexity of the possible interactions between basic hand preference, tool use, and bipedalism, and suggest that studies examining only one or two of these factors risk overlooking important patterns in the behavioral data.
Acknowledgements
The MDACC facility is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and is supported by NIH/NCRR through cooperative agreement for the NIH National Center for Research Resources. We thank the animal care technicians at MDACC for their assistance and continued support, and William Hopkins and Jamie Russell for access to unpublished data. In addition, we thank Linda Marchant, William McGrew, Steven Leigh, and an anonymous reviewer for their extremely helpful comments on earlier versions of this manuscript. Author contributions: SB and WTF designed the study, analyzed the data and wrote the paper together. SB conducted the study as part of an ongoing PhD project. SS and SL provided essential logistical support and guidance.
References
- Andrew RJ, Rogers LJ. The nature of lateralization in tetrapods. In: Rogers LJ, Andrew RJ, editors. Comparative Brain Lateralization. Cambridge University Press; Cambridge, UK: 2002. pp. 94–125. [Google Scholar]
- Annett M. Left, Right, Hand and Brain: The Right Shift Theory. LEA Publishers; London: 1985. [Google Scholar]
- Bisazza A, Cantalupo C, Robins A, Rogers LJ, Vallortigara G. Right-pawedness in toads. Nature. 1996;379:408. [Google Scholar]
- Bisazza A, Rogers LJ, Vallortigara G. The origins of cerebral asymmetry: a review of evidence of behavioral and brain lateralization in fishes, reptiles, and amphibians. Neurosci. Biobehav. Rev. 1998;22:411–426. doi: 10.1016/s0149-7634(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nettelton NC. Language lateralization to the dominant hemisphere: tool use, gesture and language in hominid evolution. Curr. Psychol. Res. Rev. 1982;2:171–192. [Google Scholar]
- Bradshaw JL, Rodgers LJ. The Evolution of Lateral Asymmetries, Language, Tool Use, and Intellect. Academic Press; San Diego, CA.: 1993. [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla g. berengei). Cortex. 1991;27:521–546. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Freeman H, Rodes W, Hopkins WD. Handedness for tool use correlated with cerebellar asymmetries in chimpanzees (Pan troglodytes). Behav. Neurosci. 2008;122:191–198. doi: 10.1037/0735-7044.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore L, Uomini N, Chapelain A. The evolution of handedness in humans and great apes: a review and current issues. J. Anthropol. Sci. 2008;86:7–35. [PubMed] [Google Scholar]
- Clapham PJ, Leimkuhler BK, Gray BK, Mattila DK. Do humpback whales exhibit lateralized behaviour? Anim. Behav. 1995;50:73–82. [Google Scholar]
- Corballis MC. Human Laterality. Academic Press; New York: 1983. [Google Scholar]
- Corballis MC. From mouth to hand: gesture, speech, and the evolution of right- handedness. Behav. Brain Sci. 2003;26:199–208. doi: 10.1017/s0140525x03000062. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C. Fifty centuries of right-handedness: the historical record. Science. 1977;198:631–632. doi: 10.1126/science.335510. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Waters NS, Denenberg AVH. The effects of early environment on the development of functional laterality in Morris maze performance. Laterality. 1997;2:221–232. doi: 10.1080/713754274. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Who forgot Paul Broca? The origin of language as test case for speciation theory. J. Linguist. 2004;41:1–24. [Google Scholar]
- D'Août K, Vereecke E, Schoonaert K, De Clercq D, Van Elsacker L, Aerts P. Locomotion in bonobos (Pan paniscus): differences and similarities between bipedal and quadrupedal terrestrial walking, and a comparison with other locomotor modes. J. Anat. 2004;204:353–361. doi: 10.1111/j.0021-8782.2004.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RA. The predatory implemental technique of Australopithecus. Am. J. Phys. Anthropol. 1949;7:1–38. doi: 10.1002/ajpa.1330070103. [DOI] [PubMed] [Google Scholar]
- Doran DM. Comparative locomotor behavior of chimpanzees and bonobos: the influence of morphology on locomotion. Am. J. Phys. Anthropol. 1993;91(1):83–98. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates. A distinction between handedness and manual specialization. Psychol. Bull. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Forsythe C, Milliken GW, Stafford DK, Ward JP. Posturally related variations in the hand preferences of the ruffed lemur (Varecia variegata variegata). J. Comp. Psychol. 1988;102:248–250. doi: 10.1037/0735-7036.102.3.248. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Ghirlanda S, Frasnelli E, Vallortigara G. Intraspecific competition and coordination in the evolution of lateralization. Philos. T. Roy. Soc. B. 2009;364:861–866. doi: 10.1098/rstb.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlanda S, Vallortigara G. The evolution of brain lateralization: a game-theoretical analysis of population structure. Proc. Roy. Soc. B. 2004;271:853–857. doi: 10.1098/rspb.2003.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KR, Ingold T. Tools, Language and Cognition in Human Evolution. Cambridge University Press; London: 1993. [Google Scholar]
- Gilbert AN, Wysocki CJ. Hand preference and age in the United States. Neuropsychology. 1992;30:601–608. doi: 10.1016/0028-3932(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Güntürkün O, Diekamp B, Manns M, Nottelmann F, Prior H, Schwarz A, Skiba M. Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 2000;10:1079–1081. doi: 10.1016/s0960-9822(00)00671-0. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Nystrom P. Handedness in captive bonobos (Pan paniscus). Folia Primatol. 2008;79:253–268. doi: 10.1159/000113539. [DOI] [PubMed] [Google Scholar]
- Heestand JE. Behavioral Lateralization in Four Species of Apes. University Microfilms International; Ann Arbor: 1986. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan troglodytes) and orangutans (Pongo pygmaeus). J. Comp. Psychol. 1993;107:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 54 years since Finch (1941). Psychol. Bull. Rev. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: statistical issues in the assessment and interpretation of hand preference data in non-human primates. Int. J. Primatol. 1999;25:1243–1265. [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychol. Bull. 2006;132:538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Handedness and neuroanatomical asymmetries in captive chimpanzees: a summary of 15 years of research. In: Hopkins WD, editor. The Evolution of Hemispheric Specialization in Primates. Elsevier Ltd.; London: 2007. pp. 59–91. [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales SL. Chimpanzee hand preference in throwing and infant cradling: implications for the origin of human handedness. Curr. Anthropol. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter AB, Pilcher DL. Grip morphology and hand use in chimpanzees (Pan troglodytes). J. Exp. Psychol. Gen. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantero M. From hand to mouth in the evolution of language: the influence of vocal behavior on lateralized hand use in manual gestures by chimpanzees (Pan troglodytes). Dev. Sci. 2003;6:55–61. [Google Scholar]
- Hopkins WD, Fernandez-Carriba S, Wesley MJ, Hostetter A, Picher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): an empirical study comparing two difference indices of laterality. J. Comp. Psychol. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Hook M, Braccini S, Schapiro SJ. Population-level right handedness for a coordinated bimanual task in chimpanzees: replication and extension in a second colony of apes. Int. J. Primatol. 2003;24:677–689. doi: 10.1023/A:1023752816951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Leavens DA. Hand use and gestural communication in chimpanzees (Pan troglodytes). J. Comp. Psychol. 1998;112:95–99. doi: 10.1037/0735-7036.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes. a review of findings. Int. J. Primatol. 1993;14:1–25. [Google Scholar]
- Hopkins WD, Russell J, Cantalupo C. Neuroanatomical correlates of handedness for tool use in chimpanzees (Pan troglodytes): implications for theories on the evolution of language. Psychol. Sci. 2007;18:971–977. doi: 10.1111/j.1467-9280.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Cantalupo C, Freeman H, Schapiro SJ. Factors influencing the prevalence and handedness for throwing in captive chimpanzees (Pan troglodytes). J. Comp. Psychol. 2005a;119:363–370. doi: 10.1037/0735-7036.119.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes). Psychol. Sci. 2005b;16:487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein EB, Henningsen H. Language lateralization in healthy right-handers. Brain. 2000a;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000b;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Larson CF, Dodson D, Ward JP. Hand preferences and whole-body turning biases of lesser bushbabies (Galago senegalensis). Brain Behav. Evol. 1989;33:261–267. doi: 10.1159/000115934. [DOI] [PubMed] [Google Scholar]
- Lazenby RA. Skeleton biology, functional asymmetry and the origins of “handedness”. J. Theor. Biol. 2002;218:129–138. doi: 10.1006/jtbi.2002.3052. [DOI] [PubMed] [Google Scholar]
- Levy J. The mammalian brain and the adaptive advantages of cerebral asymmetry. Ann. N. Y. Acad. Sci. 1977;299:264–272. doi: 10.1111/j.1749-6632.1977.tb41913.x. [DOI] [PubMed] [Google Scholar]
- Llaurens V, Raymond M, Faurie C. Why are some people left-handed? An evolutionary perspective. Philos. T. Roy. Soc. B. 2009;364:881–894. doi: 10.1098/rstb.2008.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population-level handedness for tool use. Proc. Natl. Acad. Sci. U S A. 2005;102:2634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF. The “postural origins” theory of primate neurobiological asymmetries. In: Krasnegor N, Rumbaugh D, Studdert-Kennedy M, Schiefelbusch R, editors. Biological Foundations of Language Development. Lawrence Erlbaum Associates; Hillsdale, NJ: 1991. pp. 165–188. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav. Brain Sci. 1987;15:247–303. [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema). Trends Cogn. Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Marchant LF, McGrew WC, Eibl-Eibesfeldt I. Is human handedness universal? Ethological analyses from three traditional cultures. Ethology. 1995;101:239–258. [Google Scholar]
- Martin F, Niemitz C. Right-trunkers and left-trunkers: side preferences of trunk movements in wild Asian elephants. J. Comp. Psychol. 2003;117:371–379. doi: 10.1037/0735-7036.117.4.371. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearb. Phys. Anthropol. 1997;40:201–232. [Google Scholar]
- Olson DA, Ellis JE, Nadler RD. Hand preferences in captive gorillas, orangutans, and gibbons. Am. J. Primatol. 1990;20:83–94. doi: 10.1002/ajp.1350200203. [DOI] [PubMed] [Google Scholar]
- Perelle IB, Ehrman L. An international study of human handedness: the data. Behav. Genet. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- Pollick AS, de Waal FBM. Ape gestures and language evolution. Proc. Natl. Acad. Sci. U S A. 2007;104:8184–8189. doi: 10.1073/pnas.0702624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral Preferences and Human Behavior. Springer; New York: 1981. [Google Scholar]
- Preston B. Cognition and tool use. Mind & Language 13. 1998:513–547. [Google Scholar]
- Preuschoft H, Chivers DJ. Hands of Primates. Springer-Verlag; New York, NY.: 1993. [Google Scholar]
- Pujol J, Deus J, Losilla J, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rogers LJ. Laterality in animals. Int. J. Comp. Psychol. 1989;3:5–22. [Google Scholar]
- Rogers LJ. Advantages and disadvantages of lateralization. In: Rogers LJ, Andrew JR, editors. Comparative Vertebrate Lateralization. Cambridge University Press; Cambridge: 2002. pp. 126–153. [Google Scholar]
- Rogers LJ. Lateralization in its many forms, and its evolution and development. In: Hopkins WD, editor. The Evolution of Hemispheric Specialization in Primates, Special Topics in Primatology. Elsevier; Amsterdam: 2007. pp. 23–56. [Google Scholar]
- Rogers LJ. Hand and paw preferences in relation to the lateralized brain. Philos. T. Roy. Soc. B. 2009;364:943–954. doi: 10.1098/rstb.2008.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LJ, Andrew JR. Comparative Vertebrate Lateralization. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Rogers LJ, Workman L. Footedness in birds. Anim. Behav. 1993;45:409–411. [Google Scholar]
- Roney LS, King JE. Postural effects on animal reaching laterality in squirrel monkeys (Saimiri sciureus) and cotton-top tamarins (Sanguinus oedipus). J. Comp. Psychol. 1993;107:380–385. doi: 10.1037/0735-7036.107.4.380. [DOI] [PubMed] [Google Scholar]
- Steele J, Uomini N. Humans, tools and handedness. In: Roux V, Bril B, editors. Knapping Stone: A Uniquely Hominid Behaviour. McDonald Institute for Archaeological Research; Cambridge: 2005. pp. 217–239. [Google Scholar]
- Steele J, Uomini N. Can the archeology of manual specialization tell us anything about language evolution? A survey of the state of play. Camb. Archaeol. J. 2009;19:97–110. [Google Scholar]
- Stout D. Skill and cognition in stone tool production: an ethnographic case study from Irian Jaya. Curr. Anthropol. 2002;43:693–722. [Google Scholar]
- Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool use in wild chimpanzees. Primates. 1993;34:151–159. [Google Scholar]
- Toth N. Archaeological evidence for preferential right-handedness in the lower and middle Pleistocene, and its possible implications. J. Hum. Evol. 1985;14:607–614. [Google Scholar]
- Tsai LS, Maurer S. “Right-handedness” in white rats. Science. 1930;72:436–438. doi: 10.1126/science.72.1869.436. [DOI] [PubMed] [Google Scholar]
- Vallortigara G. The evolutionary psychology of left and right: costs and benefits of lateralization. Dev. Psychobiol. 2006;48:418–427. doi: 10.1002/dev.20166. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005;28:575–633. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Walker SF. Lateralization of functions in the vertebrate brain. Br. J. Psychol. 1980;71:329–367. doi: 10.1111/j.2044-8295.1980.tb01750.x. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Kuhn HE, Suomi SJ. Effects of upright posture on hand preference for reaching vs. the use of probing tools by tufted capuchins (Cebus apella). Am. J. Primatol. 1998;44:147–153. doi: 10.1002/(SICI)1098-2345(1998)44:2<147::AID-AJP5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]