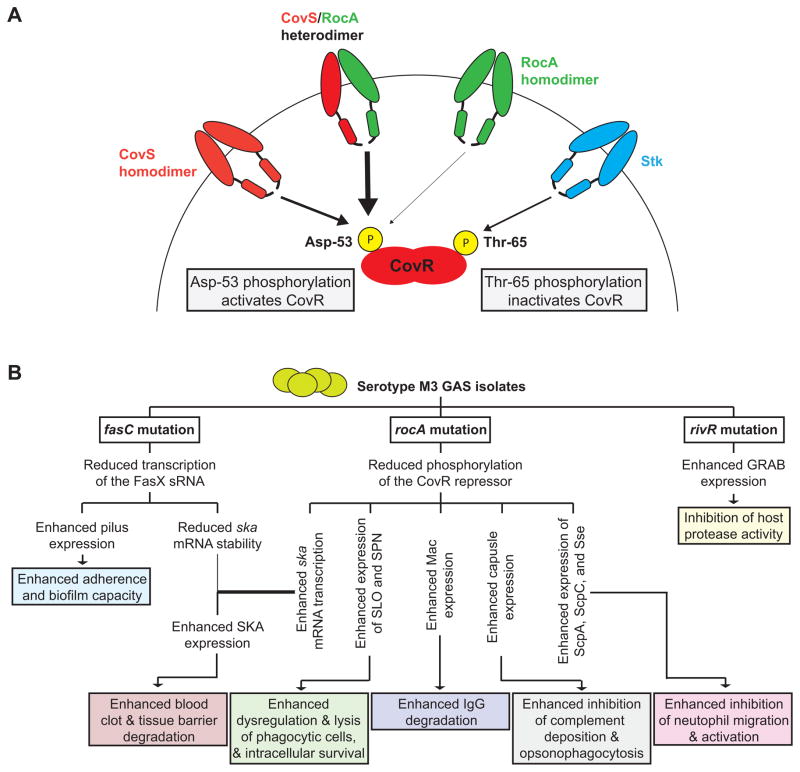
Figure 10. Models of how RocA interacts with the CovR/S system and of how the rocA mutation in M3 isolates contributes to regulatory rewiring that leads to hyper-virulence.
(A) Shown is a model of how CovS, RocA, and Stk feed into the regulation of the CovR repressor. Our data, including the finding that significant CovR phosphorylation is not observed in a covS mutant strain, are consistent with RocA promoting CovR phosphorylation on Asp-53 by forming a heterodimer with CovS, with the heterodimer having greater kinase activity than CovS or RocA homodimers. (B) Three regulatory systems are disrupted in serotype M3 GAS isolates leading to an altered virulence factor profile that, we hypothesize, is the basis behind the association of M3 isolates with severe invasive infections. The consequences of the three regulator gene mutations identified in serotype M3 GAS isolates are shown. Note that while the fasC mutation decreases streptokinase (ska) mRNA levels this is more than offset by the large increase in ska mRNA abundance as a consequence of the rocA mutation, resulting in a net increase in streptokinase expression.