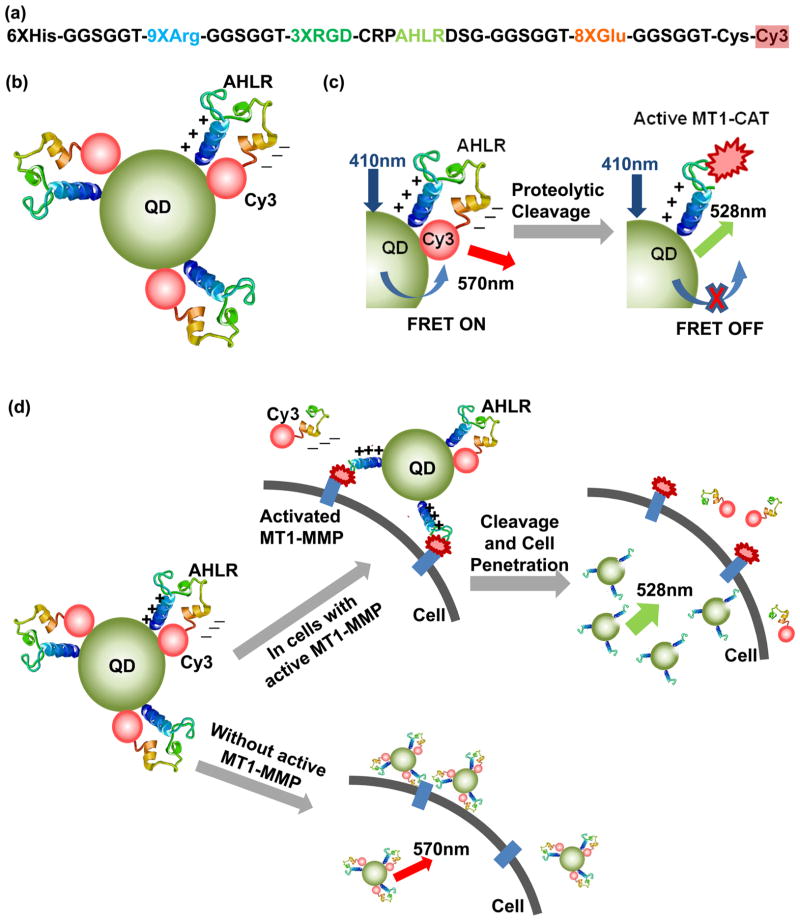
Figure 1.
Schematic illustrations of the design and activation mechanism of the QD-FRET nanosensor. (a) Designed sequence composition of a multifunctional Cy3-peptide. (b) Nanosensor contains a QD coupled to multiple Cy3-peptides bent to a position which allows a high FRET between the QD and Cy3. (c) Activation mechanism of the FRET nanosensor upon the cleavage by MT1-CAT. (d) When the sensors bind to cancer cells with active MT1-MMP, they can be cleaved at the substrate (AHLR), with decreasing FRET and increasing QD emission. Subsequently, cell penetration is triggered by the remaining positively charged peptide sequence. In addition, the nanosensors associate with the surfaces of nonmalignant cells, but they are not efficiently cleaved or and do not efficiently enter the cell.