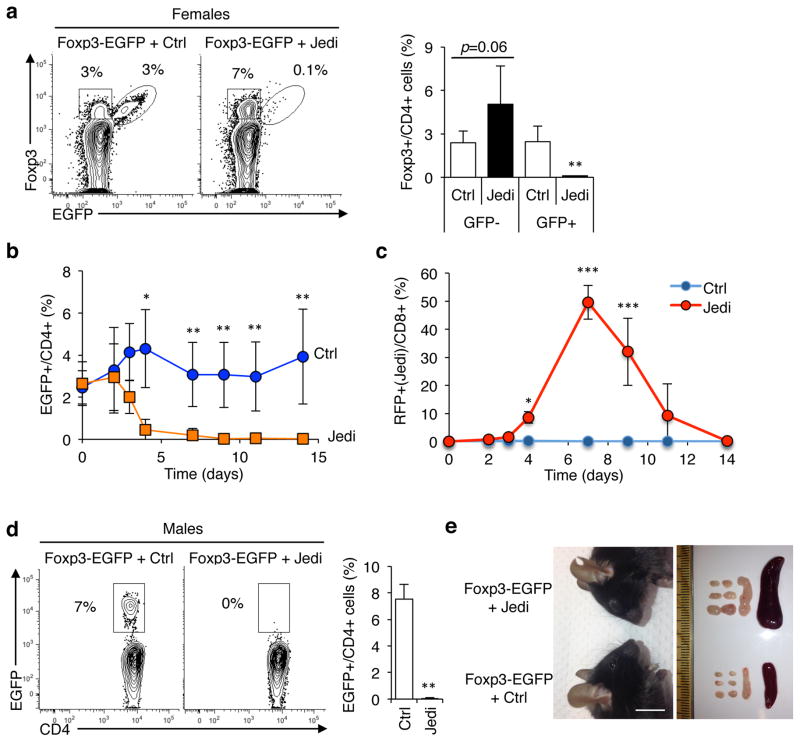
Figure 3. JEDI T-cell can stably deplete a renewing cell population to study their function.
(a) Representative flow cytometry analysis and quantification of the frequency of EGFP+ Foxp3+ cells in LN of Foxp3-EGFP females 15 days after T-cell transfer. Mice were injected with 1×106 JEDI T-cells and vaccinated immediately after. Cells were stained with CD3e, CD4, CD25 and Foxp3 to mark regulatory T-cells (n=5 mice/group). **P<0.01 vs Control-treated. (b) Foxp3-EGFP female mice were injected with either control (Ctrl) or JEDI T-cells. Percentage of EGFP+ cells among CD4+ T-cells was determined in the blood at the indicated time points (n=5 mice/group). *P<0.05 vs Control-treated, **P<0.01 vs Control-treated, ***P<0.01 vs Control-treated. (c) Percentage of JEDI T-cells among CD8+ T-cells was determined in the blood at the indicated time points from the mice described in (b) (n=5 mice/group). The JEDI mice and control littermates were transgenic actin-RFP (red florescent protein). (d) Foxp3-EGFP male mice were treated with either 5×105 control (Ctrl) or JEDI T-cells and vaccinated immediately after. Flow cytometry analysis of the frequency of EGFP+ cells in the LN 10 days after T-cell transfer. (n=3–4 mice/group). **P<0.01 vs Control-treated. (e) Representative image of the face of Foxp3-EGFP mice showing conjunctivitis in the JEDI-treated mice (left) and image of the brachial, axillary and inguinal lymph nodes and the spleen taken from control-treated and JEDI-treated Foxp3-EGFP mice 10 days after adoptive transfer (right) (n=3–4 mice/group). White bar represents 1 cm.