Figure 2.
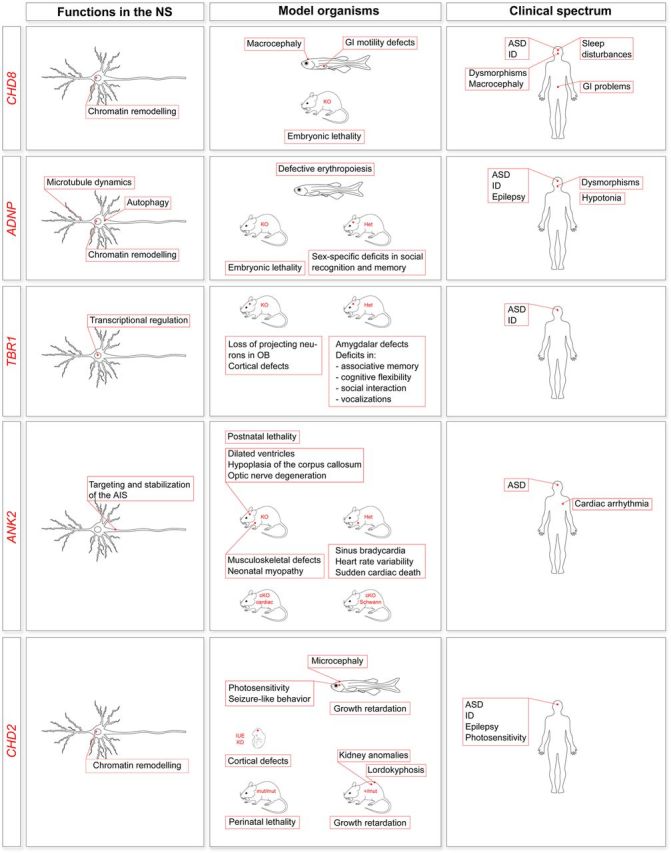
Characterization of newly discovered ASD genes. The figure summarizes, for the indicated genes, what is known about the function in the nervous system, phenotypes in animal models (KD, knockdown; KO, knockout; Het, heterozygotes) and association with human disorders. CHD8 is involved in chromatin remodeling and affects transcriptional regulation (52–54) (a postmitotic neuron is shown, but CHD8 functions in neural progenitor cells as well). In cell models, CHD8 is also implicated in apoptosis (55) (not shown). Both zebrafish (52,56) and mouse models have been generated (55,57). Chd8+/- mice have not been fully characterized. Deep phenotyping of 15 patients with CHD8 mutations has led to the identification of a proposed ASD subtype (56), although for this and many such studies the ascertainment was primarily from samples ascertained on the basis of phenotypes of interest, likely introducing biases. ADNP is involved in chromatin remodeling (58,59), autophagy (60) and microtubule dynamics at synapses through its active peptide (NAP) (61). ADNP is also expressed in glial cells (not shown) (62). ADNP knockdown in zebrafish results in no major brain anomalies but defective erythropoiesis (63). ADNP knockout in mice is embryonic lethal (64), and Adnp heterozygotes show deficits in spatial learning and memory (65) and sex-specific deficits in novel object recognition, social recognition and social memory (66). Deep phenotyping of 10 patients with ADNP mutations has led to the identification of a potential new syndrome (67). TBR1 encodes a transcription factor essential for neural stem cell fate and cerebral cortex development (68–70). TBR1 knockout in mice (71) results in the loss of projection neurons in the olfactory bulbs and olfactory cortex (71) and severe defects of cortical lamination (68,72). Tbr1+/− mice do not show gross lamination defects, but have defective axonal projections in the amygdala and a behavioral phenotype (72). TBR1 has been implicated in ASD (21,22,25,26) and ID (73). ANK2 encodes for a protein regulating the assembly of the submembranous cytoskeleton at the axonal initial segment (AIS) in unmyelinated axons (74) and acting as a glial paranodal scaffolding protein in Schwann cells (75) (not shown). ANK2 knockout in mice reduces survival postnatally (premature death by postnatal day 21) and causes lateral ventricle dilation, hypoplasia of the corpus callosum and pyramidal tracts, degeneration of the optic nerve (76) as well as musculoskeletal defects and neonatal myopathy (77). Ank2+/− mice display a cardiac phenotype (78), but their neurological phenotype has not yet been exhaustively assessed. Two conditional models have also been established: a conditional knockout of the cardiac isoforms (cKO cardiac) not fully characterized yet (79) and a knockout in Schwann cells only (cKO Schwann), with no obvious defects in paranodes of the peripheral nervous system (75). ANK2 has been associated with ASD (22,26,80) and cardiac arrhythmia (78). CHD2 is involved in chromatin remodeling [a postmitotic neuron is shown, but CHD2 functions in neural progenitor cells as well (81)]. CHD2 knockdown in zebrafish causes pericardial edema, microcephaly, body curvature, absent swim bladder, growth retardation, seizure-like behavior (82) and photosensitivity (83). CHD2 knockdown in mice by in utero electroporation causes defective cortical development by affecting neural progenitor cells proliferation (81). Homozygous mice for a CHD2 lacking the DNA-binding domain (mut/mut) have perinatal lethality (84,85). Heterozygosity (+/mut) attenuates lethality, but results in a complex phenotype (84–86). CHD2 has been associated with ASD (22,23), ID (73), epilepsy (82,87) and photosensitivity (83).
