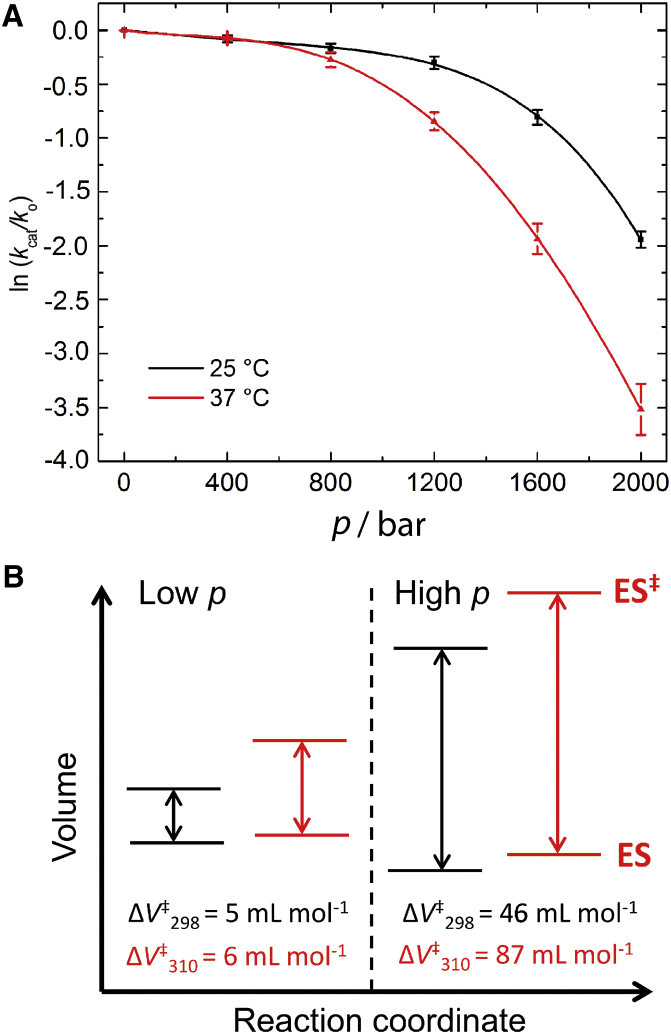
Figure 6.
(A) Pressure dependence of the enzymatic activity of MT1-MMP at 25°C (298 K) and 37°C (310 K). The value kcat/k0 corresponds the ratio between the rate constant at pressure p and the rate constant at atmospheric pressure (1 bar). The data points have been fitted to a fourth polynomial function to calculate the pressure-dependent changes in activation volume, ΔV‡cat. (B) Schematic representation of the activation volumes of the catalytic step at 25 and 37°C, respectively, displaying the difference between the volume of the transition state (ES‡) and that of the enzyme-substrate complex (ES). Each diagram represents data for the low (e.g., 400 bar, left) and the high (e.g., 1600 bar, right) pressure regime.