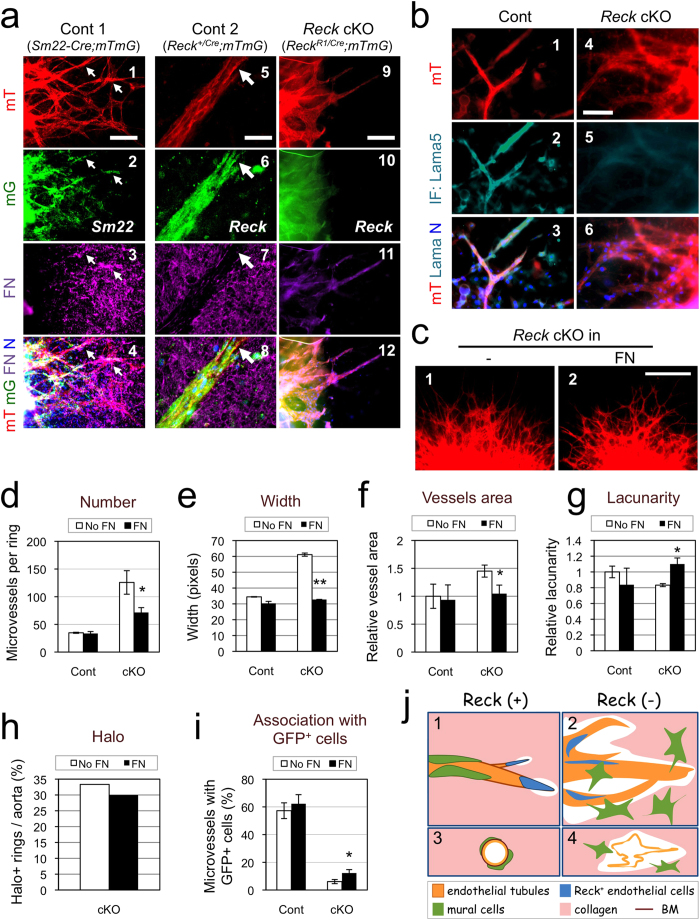
Figure 5. Functional relationship between Reck and FN in microvessel formation in ARA.
(a) Effects of Reck-deficiency on FN fibrils in ARA. Aortic rings from control 1 (Reck+/+; Sm22-Cre; mTmG), control 2 (Reck+/CrER; mTmG), and Reck cKO (ReckE1fx/CrER; mTmG) mice pretreated with tamoxifen were subjected to ARA and stained for FN (magenta, panels 3, 4, 7, 8, 11, 12) at day 10. Note that in the control samples, FN (magenta) signals are abundant around the extending sprouts (panels 3, 7), particularly accumulated in the areas where mT (red) and Sm22 (green) signals overlap (arrows in panels 1–4), but sparse near the tip of the sprouts (arrow in panels 5–8). Paucity of FN (magenta) signals in Reck cKO sample (panels 9–12) could also be observed. (b) Similar cultures stained for laminin α 5 (Lama5, turquoise). (c–i) Effects of exogenous FN on the phenotype of Reck cKO microvessels in ARA. ARAs were performed without or with addition of FN (1 μg/ml) in the gel. (c) Typical morphology of microvessels from Reck cKO aortic rings after incubation for 9 days in the absence (1) or presence (2) of exogenous FN. (d–i) Indicated parameters were measured as described in Fig. 2 a, c-f (d–h) and Fig. 4d (i) using micrographs (as shown in c) taken at day 10. Cont: n ≥ 14 rings (3 aortae); Reck cKO: n ≥ 43 rings (6 aortae). (j) A model consistent with our findings in vitro. When Reck is present (panels 1, 3), endothelial sprouting led by tip cells (blue) is appropriately regulated; tight association of mural cells (green) with endothelial tubules (orange) is promoted; and individual vessels are stabilised. When Reck is absent or reduced (panels 2, 4), endothelial sprouting and collagenolysis are activated; tip cells and mural cells localise inappropriately; and microvessels destabilise, permitting lateral fusion and ectopic anastomoses. Scale bar: (a) 20 μm in panels 5–8, 100 μm in others; (b) 50 μm; (c) 1 mm.