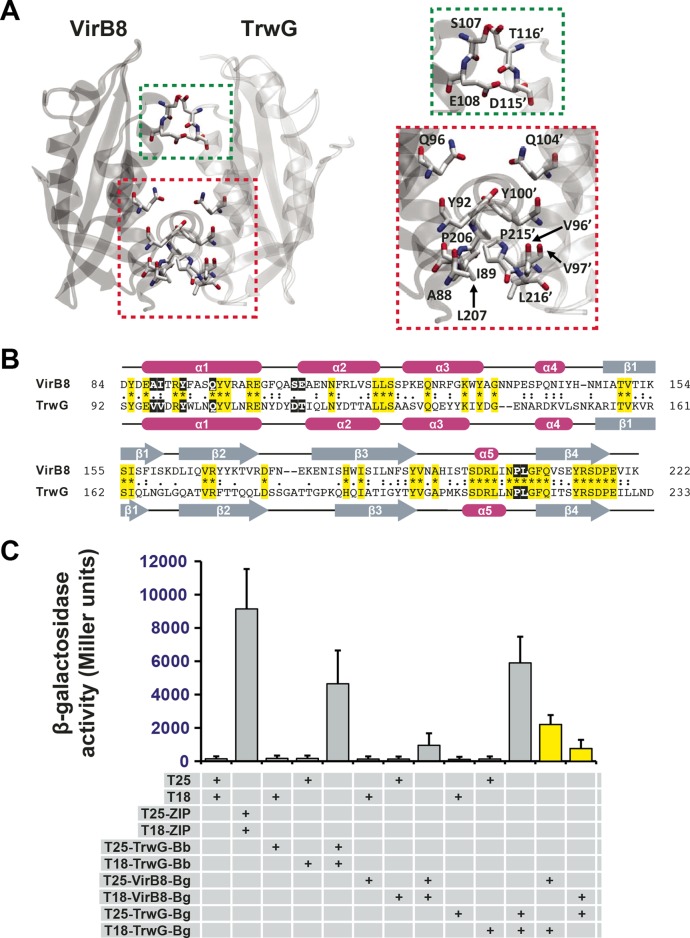
FIG 4 .
In vitro heterodimerization of Bartonella VirB8 and TrwG proteins. (A) Ribbon representation of a modeled heterodimer comprised of the VirB8 (PDB ID 4KZ1) and TrwG (PDB ID 4NHF) crystal structures from B. grahamii strain as4aup. Regions involved in dimerization are boxed and enlarged at right. Green indicates a minor dimerization site involving residues within the loop between α-helices α1 and α2. Red indicates a major dimerization site involving residues of α-helix α1 and the NPXG motif. TrwG residues are denoted with primes. (B) Sequence alignment of B. grahamii VirB8 and TrwG proteins, with secondary structure assignment. Sequences were extracted from a larger alignment (see Text S4 in the supplemental material). Sequences are the globular domains depicted in panel A. For each protein, residues involved in the dimerization interface are within black boxes. Invariant residues are highlighted in yellow. Magenta bars and gray arrows depict the α-helices and β-strands for the VirB8 and TrwG structures. (C) Analysis of B. birtlesii strain LL-WM9 and B. grahamii TrwG-TrwG interactions, the B. grahamii VirB8-VirB8 interaction, and the B. grahamii VirB8-TrwG interaction using the bacterial two-hybrid system. See the legend to Fig. 3F and the text for descriptions of the bacterial two-hybrid assay. Yellow bars depict the interactions tested for the B. grahamii VirB8-TrwG heterodimer.