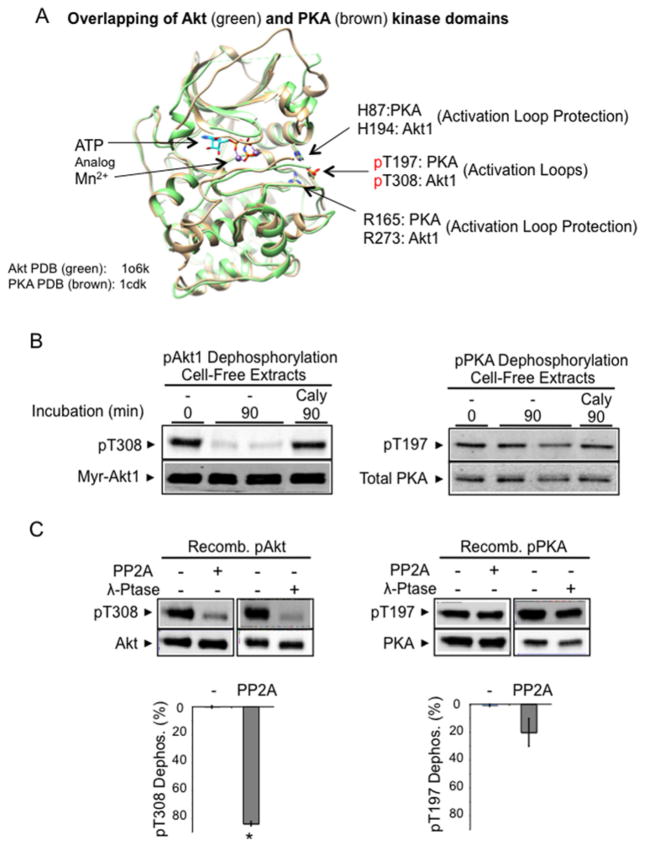
Figure 1. Constitutive dephosphorylation resistance of phospho-PKA, but not phospho-Akt, in a cell-free assay.
(A) Superimposition of Akt kinase domain (Akt2–Mn–AMP-PNP–G–SK3, green, PDB code 1o6k [28]) and PKA kinase domain (PKA–Mn–ATP–PKI, brown, PDB code 1cdk [88]). In the presence of bound ATP analogues, the phosphorylated activation loops (Thr308 in Akt1, Thr197 in PKA) shown are stabilized by invariable histidine (His194 in Akt1, His87 in PKA) and arginine (Arg273 in Akt1, Arg165 in PKA) residues. The structure was modelled on active human Akt2 crystal structures bound to the ATP analogue AMP-PNP and Mn2 + (PDB code 1o6k). Since most of our biochemical studies were performed with Akt1, we indicate the corresponding Akt1 amino acid residues in the structure for clarity. (B) Cell extract dephosphorylation of Akt and PKA. H9C2 cells expressing constitutively phosphorylated Myr-Akt1-HA and phosphorylated endogenous PKA were flash-frozen and extracted on ice. Aliquots of cell extracts without phosphatase inhibitors were incubated at 30°C for 90 min. After incubation, protein extracts were subjected to immunoblot analysis using antibodies detecting phosphorylated Akt (Thr308), HA-tagged Akt, phosphorylated PKA (Thr197) and total PKA. (C) Recombinant phospho-PKA catalytic subunit, but not that of recombinant phospho-Akt, resists dephosphorylation. Recombinant Akt1 and PKA (50 ng) were incubated with PP2A (70 ng of PP2A-C) or pan-substrate bacteriophage λ phosphatase (40 units) for 60 min at 30°C. Images show Akt1 Thr308 phosphorylation, total Akt, PKA Thr197 phosphorylation and total PKA. The histograms show the percentage of pThr308 or pThr197 dephosphorylation. n=4/group. *P < 0.05 compared with PKA dephosphorylation by PP2A.