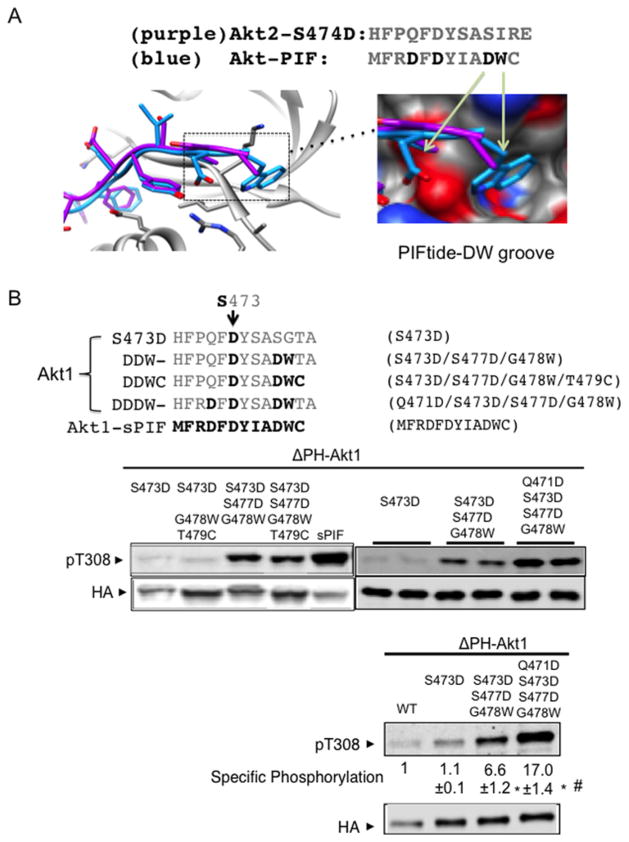
Figure 4. Progressive protection of Akt dephosphorylation by a DDDW targeting groove.
(A) Akt structures were superimposed with Akt C-terminal amino acids (purple) or with PIFtide amino acids (blue). Image depicts the insertion of PIFtide DW residues into an Akt groove. The structure shown is modelled on active human Akt2 crystal structures bound to the ATP analogue AMP-PNP and Mn2 + with S474D mutation (PDB code 1o6k) or with PIFtide fusion (PDB code 1o6l). Most of our biochemical studies were performed with Akt1. When Akt2 structure models were used, we indicate the corresponding Akt1 amino acid residues for clarity. (B) The C-terminally modified cytosolic Akt (ΔPH-Akt1) constructs were transfected into HEK293 cells and incubated for 4 h in medium that was free of exogenous growth factors. Protein extracts were subjected to immunoblot analysis using antibodies to phospho-Akt Thr308 and anti-HA. For quantification, we used a two-colour infrared fluorescence quantitative Western blot system with Li-Cor Odyssey imager to measure band intensity from different blots. In each blot, pThr308 signal and the HA signal in ΔPH-Akt1-WT were normalized to 1. The specific phosphorylation is the ratio of the normalized pThr308 signal to normalized HA signals. *P < 0.001 ΔPH-Akt1-DDW (n=9) or ΔPH-Akt1-DDDW (n=11) compared with ΔPH-Akt1-S473D (n=8). #P < 0.001 ΔPH-Akt1-DDW compared with ΔPH-Akt1-DDDW.