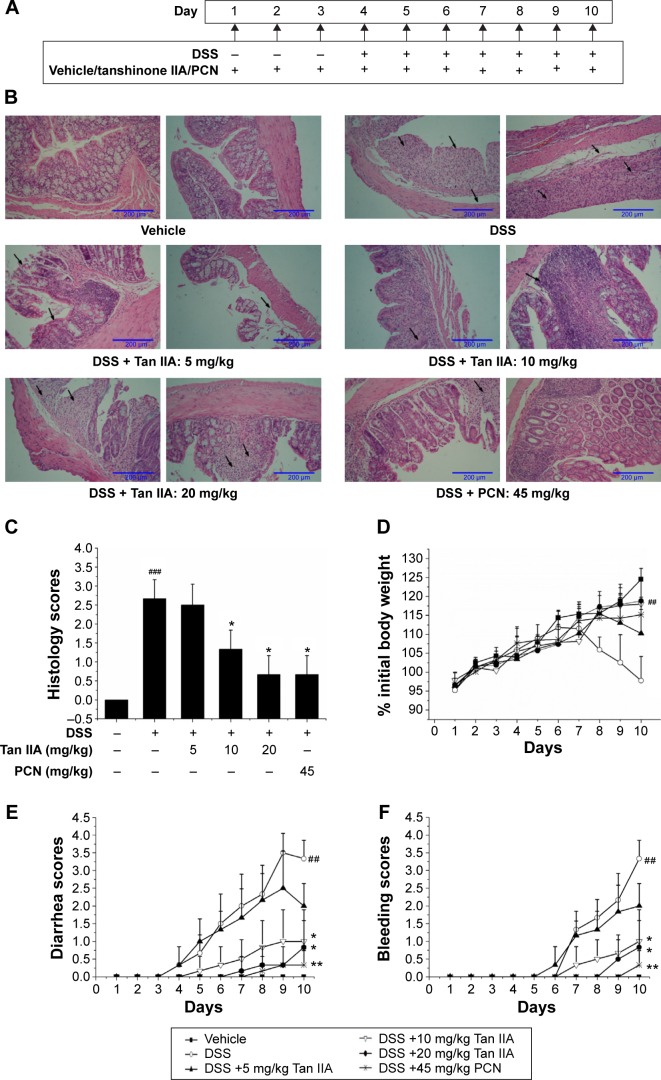
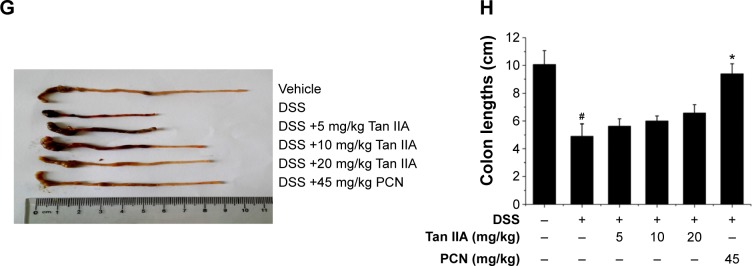
Figure 4.
Clinical assessment of dextran sulfate sodium (DSS)-induced inflammatory bowel disease in vehicle-, Tan IIA-, and PCN-treated mouse model.
Notes: (A) Dosing scheme; (B) Representative hematoxylin and eosin stained colon sections (left 20×, right 40× magnification); areas of severe colon necrosis are marked with arrows; (C) Histology scores, as assessed according to morphological criteria and the proportion of the colon that was affected by the individual changes relative to the total area of the colon section; (D) Changes in body weight after DSS induction of colitis; (E) Diarrhea scores; (F) Bleeding scores; (G) Macroscopic observation of colon length; (H) Colon lengths; DSS-stimulated elevated histology scores, diarrhea, and bleeding while the body weight and colon lengths were significantly reduced by cotreatment with different doses of Tan IIA or 45 mg/kg PCN; the injury has been ameliorated in dose-dependent manner; Each bar represents the means ± SD (n=10). *P<0.01, **P<0.001 versus DSS-treated group. #P<0.05, ##P<0.01, ###P<0.001 versus vehicle-treated group.
Abbreviations: PCN, pregnenolone 16a-carbonitrile; SD, standard deviation; Tan IIA, tanshinone IIA.